Abstract
Background:
4-1BB, a member of the TNF receptor superfamily, has a role in various inflammatory pathologies through its interaction with 4-1BB ligand. We previously demonstrated that it participates in initiating and promoting obesity-induced adipose inflammation in a rodent model.
Objective:
In this study, we examined whether 4-1BB is related to obesity-induced adipose inflammation and metabolic parameters in humans.
Methods:
A total of 50 subjects, 25 obese (body mass index (BMI)⩾25 kg m−2) and 25 lean (BMI<23 kg m−2) participated in the study. The levels of 4-1BB transcripts and soluble 4-1BB protein (s4-1BB) in subcutaneous adipose tissue were measured by quantitative real-time PCR and enzyme-linked immunosorbent assay, respectively. Inflammatory and metabolic parameters were measured by enzymatic analysis and immunoassay.
Results:
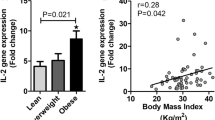
Obese subjects had higher levels of both 4-1BB transcripts and s4-1BB protein in subcutaneous adipose tissue than lean controls, and the levels were correlated with BMI and the expression of inflammatory markers, as well as with serum metabolic parameters. Moreover, s4-1BB was released from human adipocytes, and elicited chemotactic responses from human monocytes/T cells as well as enhancing their inflammatory activity, indicating that it may promote human adipose inflammation.
Discussion:
Our data demonstrate that elevated levels of 4-1BB transcripts and s4-1BB in adipose tissue are closely associated with obesity-induced inflammation and metabolic dysregulation. They suggest that both 4-1BB transcripts and s4-1BB could serve as novel biomarkers and/or therapeutic targets for obesity-induced inflammation and metabolic syndrome in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM . Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995; 95: 2409.
Rasouli N, Kern PA . Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 2008; 93: s64–s73.
Grundy SM . Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 2004; 89: 2595–2600.
Wisse BE . The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004; 15: 2792–2800.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Shoelson SE, Lee J, Goldfine AB . Inflammation and insulin resistance. J Clin Invest 2006; 116: 1793–1801.
Suganami T, Nishida J, Ogawa YA . Paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor α. Arterioscler Thromb Vac Biol 2005; 25: 2062–2068.
Poggi M, Jager J, Paulmyer-Lacroix O, Peiretti F, Gremeaux T, Verdier M et al. The inflammatory receptor CD40 is expressed on human adipocytes: contribution to crosstalk between lymphocytes and adipocytes. Diabetologia 2009; 52: 1152–1163.
Tu TH, Kim C-S, Goto T, Kawada T, Kim B-S, Yu R . 4-1BB/4-1BBL interaction promotes obesity-induced adipose inflammation by triggering bidirectional inflammatory signaling in adipocytes/macrophages. Mediators Inflamm 2012; 2012: 972629.
Brake DK, Smith EB, Mersmann H, Smith CW, Robker RL . ICAM-1 expression in adipose tissue: effects of diet-induced obesity in mice. Am J Physiol Cell Physiol 2006; 291: C1232–C1239.
Lumeng CN, Maillard I, Saltiel AR . T-ing up inflammation in fat. Nat Med 2009; 15: 846–847.
Kim HM, Jeong CS, Choi HS, Kawada T, Yu R . LIGHT/TNFSF14 enhances adipose tissue inflammatory responses through its interaction with HVEM. FEBS Lett 2011; 33: 144–152.
Bassols J, Moreno JM, Ortega F, Ricart W, Fernandez-Real JM . Characterization of herpes virus entry mediator as a factor linked to obesity. Obesity 2010; 18: q239–246.
Metkar SS, Naresh K, Manna PP, Srinivas V, Advani S, Nadkarni J . Circulating levels of TNFα and TNF receptor superfamily members in lymphoid neoplasia. Am J Hematol 2000; 65: 105–110.
Seijkens T, Kusters P, Engel D, Lutgens E . CD40–CD40L: linking pancreatic, adipose tissue and vascular inflammation in type 2 diabetes and its complications. Diab Vasc Dis Res 2013; 10: 115–122.
Desideri G, Ferri C . Effects of obesity and weight loss on soluble CD40L levels. JAMA 2003; 289: 1781–1782.
Missiou A, Wolf D, Platzer I, Ernst S, Walter C, Rudolf P et al. CD40L induces inflammation and adipogenesis in adipose cells-a potential link between metabolic and cardiovascular disease. Thromb Haemost 2010; 103: 788.
Vinay DS, Choi BK, Bae JS, Kim WY, Gebhardt BM, Kwon BS . CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J Immunol 2004; 173: 4218–4229.
Kim D-H, Chang W-S, Lee Y-S, Lee K-A, Kim Y-K, Kwon BS et al. 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol 2008; 180: 2062–2068.
Kim C-S, Kim JG, Lee B-J, Choi M-S, Choi H-S, Kawada T et al. Deficiency for costimulatory receptor 4-1BB protects against obesity-induced inflammation and metabolic disorders. Diabetes 2011; 60: 3159–3168.
Shao Z, Schwarz H . CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol 2011; 89: 21–29.
Lippert U, Zachmann K, Ferrari DM, Schwarz H, Brunner E, Latif A et al. CD137 ligand reverse signaling has multiple functions in human dendritic cells during an adaptive immune response. Eur J Immunol 2008; 38: 1024–1032.
Jeon HJ, Choi J-H, Jung I-H, Park J-G, Lee M-R, Lee M-N et al. CD137 (4–1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation 2010; 121: 1124–1133.
Seo SK, Park HY, Choi JH, Kim WY, Kim YH, Jung HW et al. Blocking 4-1BB/4-1BB ligand interactions prevents herpetic stromal keratitis. J Immunol 2003; 171: 576–583.
Kwon B . CD137-CD137 ligand interactions in inflammation. Immune Netw 2009; 9: 84–89.
Shao Z, Schäffler A, Hamer O, Dickopf J, Goetz A, Landfried K et al. Admission levels of soluble CD137 are increased in patients with acute pancreatitis and are associated with subsequent complications. Exp Mol Pathol 2012; 92: 1–6.
Dongming L, Zuxun L, Liangjie X, Biao W, Ping Y . Enhanced levels of soluble and membrane-bound CD137 levels in patients with acute coronary syndromes. Clin Chim Acta 2010; 411: 406–410.
Jung HW, Choi SW, Choi JI, Kwon BS . Serum concentrations of soluble 4-1BB and 4-1BB ligand correlated with the disease severity in rheumatoid arthritis. Exp Mol Med 2004; 36: 13–22.
Michel J, Langstein J, Hofstädter F, Schwarz H . A soluble form of CD137 (ILA/4‐1BB), a member of the TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur J Immunol 1998; 28: 290–295.
Sharief M . Heightened intrathecal release of soluble CD137 in patients with multiple sclerosis. Eur J Neurol 2002; 9: 49–54.
Yan J, Gong J, Liu P, Wang C, Chen G . Positive correlation between CD137 expression and complex stenosis morphology in patients with acute coronary syndromes. Clin Chim Acta 2011; 412: 993–998.
Yan J, Wang C, Chen R, Yang H . Clinical implications of elevated serum soluble CD137 levels in patients with acute coronary syndrome. Clinics 2013; 68: 193.
Organization WH. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. World Health Organization: Geneva, Switzerland, 2000.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502.
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R . Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Alberti KGMM Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645.
Alberti K, Zimmet P, Shaw J . Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006; 23: 469–480.
Shao Z, Schäffler A, Hamer O, Dickopf J, Goetz A, Landfried K et al. Admission levels of soluble CD137 are increased in patients with acute pancreatitis and are associated with subsequent complications. Exp Mol Pathol 2011; 92: 1–6.
Olsson I, Gatanaga T, Gullberg U, Lantz M, Granger G . Tumour necrosis factor (TNF) binding proteins (soluble TNF receptor forms) with possible roles in inflammation and malignancy. Eur Cytokine Netw 1993; 4: 169.
Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM . The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J 2001; 15: 43–58.
Heaney ML, Golde DW . Soluble receptors in human disease. J Leukoc Biol 1998; 64: 135–146.
Kim W, Kim J, Jung D, Kim H, Choi H-J, Cho HR et al. Induction of lethal graft-versus-host disease by anti-CD137 monoclonal antibody in mice prone to chronic graft-versus-host disease. Biol Blood Marrow Transplant 2009; 15: 306–314.
Cartier A, Côté M, Bergeron J, Alméras N, Tremblay A, Lemieux I et al. Plasma soluble tumour necrosis factor-α receptor 2 is elevated in obesity: specific contribution of visceral adiposity. Clin Endocrinol (Oxf) 2010; 72: 349–357.
Choi JW, Kim SK . Relationships of soluble APO-1 (Fas/CD95) concentrations, obesity, and serum lipid parameters in healthy adults. Ann Clin Lab Sci 2005; 35: 290–296.
Anderson DR, Poterucha JT, Mikuls TR, Duryee MJ, Garvin RP, Klassen LW et al. IL-6 and its receptors in coronary artery disease and acute myocardial infarction. Cytokine 2013; 62: 395–400.
Michel J, Schwarz H . Expression of soluble CD137 correlates with activation-induced cell death of lymphocytes. Cytokine 2000; 12: 742–746.
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15: 914–920.
Salih HR, Schmetzer HM, Burke C, Starling GC, Dunn R, Pelka-Fleischer R et al. Soluble CD137 (4-1BB) ligand is released following leukocyte activation and is found in sera of patients with hematological malignancies. J Immunol 2001; 167: 4059–4066.
Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF . Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci USA 1992; 89: 4845–4849.
Hirano T . Interleukin 6 and its receptor: ten years later. Int Rev Immunol 1998; 16: 249–284.
Furtner M, Straub R, Krüger S, Schwarz H . Levels of soluble CD137 are enhanced in sera of leukemia and lymphoma patients and are strongly associated with chronic lymphocytic leukemia. Leukemia 2005; 19: 883–885.
Olofsson PS, Söderström LÅ, Wågsäter D, Sheikine Y, Ocaya P, Lang F et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation 2008; 117: 1292–1301.
He M, Li ETS, Harris S, Huff MW, Yau CY, Anderson GH . Canadian global village reality: anthropometric surrogate cutoffs and metabolic abnormalities among Canadians of East Asian, South Asian, and European descent. Can Fam Physician 2010; 56: e174–e182.
Rakugi H, Ogihara T . The metabolic syndrome in the Asian population. Curr Sci Inc 2005; 7: 103–109.
Acknowledgements
This work has supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No.2012R1A2A4A01002702), and an NRF grant funded by the Korean government (MSIP) (No.2008-0062618).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Tu, T., Kim, CS., Kang, JH. et al. Levels of 4-1BB transcripts and soluble 4-1BB protein are elevated in the adipose tissue of human obese subjects and are associated with inflammatory and metabolic parameters. Int J Obes 38, 1075–1082 (2014). https://doi.org/10.1038/ijo.2013.222
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.222
Keywords
This article is cited by
-
Soluble CD137 and risk of hepatocellular carcinoma: nested case–control studies in cohorts in Shanghai and Singapore
British Journal of Cancer (2023)
-
Transcriptome of CD8+ tumor-infiltrating T cells: a link between diabetes and colorectal cancer
Cancer Immunology, Immunotherapy (2021)
-
Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9
Scientific Reports (2018)