Abstract
Background:
Cues that are associated with the availability of food are known to trigger food anticipatory activity (FAA). This activity is expressed as increased locomotor activity and enables an animal to prepare for maximal utilization of nutritional resources. Although the exact neural network that mediates FAA is still unknown, several studies have revealed that the medial hypothalamus is involved. Interestingly, this area is responsive to the anorexigenic hormone leptin and the orexigenic hormone ghrelin that have been shown to modulate FAA. However, how FAA is regulated by neuronal activity and how leptin and ghrelin modulate this activity is still poorly understood.
Objective:
We aimed to examine how the total neuronal population and individual neurons in the medial hypothalamus respond to cue-signaled food availability in awake, behaving rats. In addition, ghrelin and leptin were injected to investigate whether these hormones could have a modulatory role in the regulation of FAA.
Design:
Using in vivo electrophysiology, neuronal activity was recorded in the medial hypothalamus in freely moving rats kept on a random feeding schedule, in which a light cue signaled upcoming food delivery. Ghrelin and leptin were administered systemically following the behavioral paradigm.
Results:
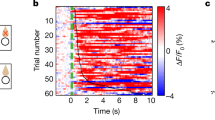
The food-predictive cue induced FAA as well as a significant increase in neural activity on a population level. More importantly, a sub-population of medial hypothalamic neurons displayed highly correlated identical responses to both ghrelin and FAA, suggesting that these neurons are part of the network that regulates FAA.
Conclusion:
This study reveals a role for ghrelin, but not leptin, signaling within medial hypothalamus in FAA on both a population level and in single cells, identifying a subset of neurons onto which cue information and ghrelin signaling converge, possibly to drive FAA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mistlberger RE . Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 1994; 18: 171–195.
Schiltz CA, Bremer QZ, Landry CF, Kelley AE . Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol 2007; 5: 16.
Barbano MF, Cador M . Various aspects of feeding behavior can be partially dissociated in the rat by the incentive properties of food and the physiological state. Behav Neurosci 2005; 119: 1244–1253.
Petrovich GD, Holland PC, Gallagher M . Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci 2005; 25: 8295–8302.
Davidson AJ . Lesion studies targeting food-anticipatory activity. Eur J Neurosci 2009; 30: 1658–1664.
Gooley JJ, Schomer A, Saper CB . The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci 2006; 9: 398–407.
Angeles-Castellanos M, Aguilar-Roblero R, Escobar C . c-Fos expression in hypothalamic nuclei of food-entrained rats. Am J Physiol Regul Integr Comp Physiol 2004; 286: R158–R165.
Ribeiro AC, Sawa E, Carren-LeSauter I, LeSauter J, Silver R, Pfaff DW . Two forces for arousal: pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci USA 2007; 104: 20078–20083.
Acosta-Galvan G, Yi CX, van der Vliet J, Jhamandas JH, Panula P, Angeles-Castellanos M et al. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc Natl Acad Sci USA 2011; 108: 5813–5818.
Tahara Y, Hirao A, Moriya T, Kudo T, Shibata S . Effects of medial hypothalamic lesions on feeding-induced entrainment of locomotor activity and liver Per2 expression in Per2::luc mice. J Biol Rhythms 2010; 25: 9–18.
Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE . The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms 2007; 22: 467–478.
Landry GJ, Simon MM, Webb IC, Mistlberger RE . Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol 2006; 290: R1527–R1534.
Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N et al. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur J Neurosci 2009; 29: 1447–1460.
Krieger DT . Ventromedial hypothalamic lesions abolish food-shifted circadian adrenal and temperature rhythmicity. Endocrinology 1980; 106: 649–654.
Inouye ST . Ventromedial hypothalamic lesions eliminate anticipatory activities of restricted daily feeding schedules in the rat. Brain Res 1982; 250: 183–187.
Mistlberger RE, Rechtschaffen A . Recovery of anticipatory activity to restricted feeding in rats with ventromedial hypothalamic lesions. Physiol Behav 1984; 33: 227–235.
Honma S, Honma K, Nagasaka T, Hiroshige T . The ventromedial hypothalamic nucleus is not essential for the prefeeding corticosterone peak in rats under restricted daily feeding. Physiol Behav 1987; 39: 211–215.
Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG . Identification of targets of leptin action in rat hypothalamus. J Clin Invest 1996; 98: 1101–1106.
Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 1997; 48: 23–29.
Verhagen LA, Egecioglu E, Luijendijk MC, Hillebrand JJ, Adan RA, Dickson SL . Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur Neuropsychopharmacol 2011; 21: 384–392.
Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL et al. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience 2009; 164: 351–359.
LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R . Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA 2009; 106: 13582–13587.
Davis JF, Choi DL, Clegg DJ, Benoit SC . Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiol Behav 2010; 103: 39–43.
Ribeiro AC, Ceccarini G, Dupre C, Friedman JM, Pfaff DW, Mark AL . Contrasting effects of leptin on food anticipatory and total locomotor activity. PLoS One 2011; 6: e23364.
Mistlberger RE, Marchant EG . Enhanced food-anticipatory circadian rhythms in the genetically obese Zucker rat. Physiol Behav 1999; 66: 329–335.
Persons JE, Stephan FK, Bays ME . Diet-induced obesity attenuates anticipation of food access in rats. Physiol Behav 1993; 54: 55–64.
Thompson RH, Swanson LW . Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Brain Res Rev 1998; 27: 89–118.
Thompson RH, Canteras NS, Swanson LW . Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol 1996; 376: 143–173.
Yoshida K, Li X, Cano G, Lazarus M, Saper CB . Parallel preoptic pathways for thermoregulation. J Neurosci 2009; 29: 11954–11964.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates 5th edn Elsevier: Amsterdam, The Netherlands, 2005.
Escobar C, Martinez-Merlos MT, Angeles-Castellanos M, del Carmen MM, Buijs RM . Unpredictable feeding schedules unmask a system for daily resetting of behavioural and metabolic food entrainment. Eur J Neurosci 2007; 26: 2804–2814.
Kobelt P, Wisser AS, Stengel A, Goebel M, Inhoff T, Noetzel S et al. Peripheral injection of ghrelin induces Fos expression in the dorsomedial hypothalamic nucleus in rats. Brain Res 2008; 1204: 77–86.
Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000; 141: 4325–4328.
Ruter J, Kobelt P, Tebbe JJ, Avsar Y, Veh R, Wang L et al. Intraperitoneal injection of ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus in rats. Brain Res 2003; 991: 26–33.
Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA . Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol 2002; 14: 580–586.
Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ . Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci USA 1999; 96: 7047–7052.
Caquineau C, Douglas AJ, Leng G . Effects of cholecystokinin in the supraoptic nucleus and paraventricular nucleus are negatively modulated by leptin in 24-h fasted lean male rats. J Neuroendocrinol 2010; 22: 446–452.
van Duuren E, van der Plasse G, van der Blom R, Joosten RN, Mulder AB, Pennartz CM et al. Pharmacological manipulation of neuronal ensemble activity by reverse microdialysis in freely moving rats: a comparative study of the effects of tetrodotoxin, lidocaine, and muscimol. J Pharmacol Exp Ther 2007; 323: 61–69.
Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB . Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 1998; 395: 535–547.
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK . Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 2006; 494: 528–548.
Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol 2000; 423: 261–281.
Niimi M, Sato M, Yokote R, Tada S, Takahara J . Effects of central and peripheral injection of leptin on food intake and on brain Fos expression in the Otsuka Long-Evans Tokushima Fatty rat with hyperleptinaemia. J Neuroendocrinol 1999; 11: 605–611.
Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB . Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA 1998; 95: 741–746.
van Dijk G, Thiele TE, Donahey JC, Campfield LA, Smith FJ, Burn P et al. Central infusions of leptin and GLP-1-(7-36) amide differentially stimulate c-FLI in the rat brain. Am J Physiol 1996; 271: R1096–R1100.
Solomon A, De Fanti BA, Martinez JA . Peripheral ghrelin participates in the glucostatic signaling mediated by the ventromedial and lateral hypothalamus neurons. Peptides 2006; 27: 1607–1615.
Lawrence CB, Snape AC, Baudoin FM, Luckman SM . Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 2002; 143: 155–162.
Hillebrand JJ, Koeners MP, de Rijke CE, Kas MJ, Adan RA . Leptin treatment in activity-based anorexia. Biol Psychiatry 2005; 58: 165–171.
Hillebrand JJ, Kas MJ, van Elburg AA, Hoek HW, Adan RA . Leptin’s effect on hyperactivity: potential downstream effector mechanisms. Physiol Behav 2008; 94: 689–695.
Hebebrand J, Exner C, Hebebrand K, Holtkamp C, Casper RC, Remschmidt H et al. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiol Behav 2003; 79: 25–37.
Sabatier N, Leng G . Spontaneous discharge characteristic of neurons in the ventromedial nucleus of the rat hypothalamus in vivo. Eur J Neurosci 2008; 28: 693–706.
Chen X, Ge YL, Jiang ZY, Liu CQ, Depoortere I, Peeters TL . Effects of ghrelin on hypothalamic glucose responding neurons in rats. Brain Res 2005; 1055: 131–136.
Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 2006; 49: 191–203.
Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML . Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 1997; 390: 521–525.
Yanagida H, Morita T, Kim J, Yoshida K, Nakajima K, Oomura Y et al. Effects of ghrelin on neuronal activity in the ventromedial mucleus of the hypothalamus in infantile rats: an in vitro study. Peptides 2008; 29: 912–918.
Hewson AK, Viltart O, McKenzie DN, Dyball RE, Dickson SL . GHRP-6-induced changes in electrical activity of single cells in the arcuate, ventromedial and periventricular nucleus neurons of a hypothalamic slice preparation in vitro. J Neuroendocrinol 1999; 11: 919–923.
Kumarnsit E, Johnstone LE, Leng G . Actions of neuropeptide Y and growth hormone secretagogues in the arcuate nucleus and ventromedial hypothalamic nucleus. Eur J Neurosci 2003; 17: 937–944.
Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P . Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 1995; 269: 546–549.
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995; 269: 543–546.
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001; 120: 337–345.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K . Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999; 402: 656–660.
Tschop M, Smiley DL, Heiman ML . Ghrelin induces adiposity in rodents. Nature 2000; 407: 908–913.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DSA . preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714–1719.
Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC . Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 2006; 147: 23–30.
Schüssler P, Kluge M, Yassouridis A, Dresler M, Uhr M, Steiger A . Ghrelin levels increase after pictures showing food. Obesity 20: 1212–1217.
Acknowledgements
The current work was supported by the EU (FP7-KBBE-2009-3-245009 (NeuroFAST) and FP7-KBBE-2010-4-266408 (Full4Health)) and NWO Toptalent.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
van der Plasse, G., Merkestein, M., Luijendijk, M. et al. Food cues and ghrelin recruit the same neuronal circuitry. Int J Obes 37, 1012–1019 (2013). https://doi.org/10.1038/ijo.2012.174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.174
Keywords
This article is cited by
-
Integrative Functions of the Cortico-Strio-Thalamo-Cortical System of the Brain
Neuroscience and Behavioral Physiology (2022)
-
Impact of sleeve gastrectomy compared to Roux-en-y gastric bypass upon hedonic hunger and the relationship to post-operative weight loss
Internal and Emergency Medicine (2022)
-
Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin
International Journal of Obesity (2015)
-
GHS-R1a signaling in the DMH and VMH contributes to food anticipatory activity
International Journal of Obesity (2014)