Abstract
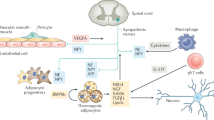
The brown adipocyte is a thermogenic cell. Its thermogenic potential is conferred by uncoupling protein-1, which ‘uncouples’ adenosine triphosphate synthesis from energy substrate oxidation. Brown fat cells in so-called classical brown adipose tissue (BAT) share their origin with myogenic factor-5-expressing myoblasts. The development of myocyte/brown adipocyte progenitor cells into a brown adipocyte lineage is apparently triggered by bone morphogenetic protein-7, which stimulates inducers of brown fat cell differentiation, such as PRD1-BF1-RIZ1 homologous domain-containing-16 and peroxisome proliferator-activated receptor-γ co-activator-1-α. The control of brown fat cell development and activity is physiologically ensured by the sympathetic nervous system (SNS), which densely innervates BAT. SNS-mediated thermogenesis is largely governed by hypothalamic and brainstem neurons. With regard to energy balance, the leptin–melanocortin pathway appears to be a major factor in controlling brown adipocyte thermogenesis. The involvement of this homeostatic pathway further supports the role of the brown adipocyte in energy balance regulation. The interest for the brown fat cell and its potential role in energy balance has been further rejuvenated recently by the demonstration that BAT can be present in substantial amounts in humans, in contrast to what has always been thought. Positron emission tomography/computed tomography scanning investigations have indeed revealed the presence in humans of important neck and shoulder cold-activable BAT depots, in particular, in young, lean and female subjects. This short review summarizes recent progress made in the biology of the brown fat cell and focuses on the determinants of the brown adipocyte development and activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gesner C . Medici Tigurini Historiae Animalium Liber I de Quadrupedibusuiuiparis.
Klingenspor M . Cold-induced recruitment of brown adipose tissue thermogenesis. Exp Physiol 2003; 88: 141–148.
Himms-Hagen J . Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J 1990; 4: 2890–2898.
Foster DO, Frydman ML . Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol 1979; 57: 257–270.
Ricquier D . Respiration uncoupling and metabolism in the control of energy expenditure. Proc Nutr Soc 2005; 64: 47–52.
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359.
Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009; 23: 3113–3120.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508.
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
Cohade C, Mourtzikos KA, Wahl RL . ‘USA-Fat’: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med 2003; 44: 1267–1270.
Cohade C, Osman M, Pannu HK, Wahl RL . Uptake in supraclavicular area fat (″USA-Fat″): description on 18F-FDG PET/CT. J Nucl Med 2003; 44: 170–176.
Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 2006; 296: 164–176.
Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab 2007; 6: 38–54.
Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA 2007; 104: 4401–4406.
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454: 1000–1004.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454: 961–967.
Morrison SF, Nakamura K, Madden CJ . Central control of thermogenesis in mammals. Exp Physiol 2008; 93: 773–797.
Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ . Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol 2008; 295: R417–R428.
Bartness TJ, Song CK . Brain-adipose tissue neural crosstalk. Physiol Behav 2007; 91: 343–351.
Sell H, Deshaies Y, Richard D . The brown adipocyte: update on its metabolic role. Int J Biochem Cell Biol 2004; 36: 2098–2104.
Cinti S . Reversible physiological transdifferentiation in the adipose organ. Proc Nutr Soc 2009; 68: 340–349.
Cinti S . The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc 2001; 60: 319–328.
Cinti S . Transdifferentiation properties of adipocytes in the Adipose Organ. Am J Physiol Endocrinol Metab 2009; 297: E977–E986.
Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009; 460: 1154–1158.
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM . A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92: 829–839.
Wu Z, Boss O . Targeting PGC-1 alpha to control energy homeostasis. Expert Opin Ther Targets 2007; 11: 1329–1338.
Karamanlidis G, Karamitri A, Docherty K, Hazlerigg DG, Lomax MA . C/EBPbeta reprograms white 3T3-L1 preadipocytes to a Brown adipocyte pattern of gene expression. J Biol Chem 2007; 282: 24660–24669.
Tontonoz P, Spiegelman BM . Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 2008; 77: 289–312.
Lefterova MI, Lazar MA . New developments in adipogenesis. Trends Endocrinol Metab 2009; 20: 107–114.
Fruhbeck G, Becerril S, Sainz N, Garrastachu P, Garcia-Velloso MJ . BAT: a new target for human obesity? Trends Pharmacol Sci 2009; 30: 387–396.
Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M . Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab 2009; 10: 324–335.
Walden TB, Timmons JA, Keller P, Nedergaard J, Cannon B . Distinct expression of muscle-specific microRNAs (myomirs) in brown adipocytes. J Cell Physiol 2009; 218: 444–449.
Cannon B, Nedergaard J . Developmental biology: neither fat nor flesh. Nature 2008; 454: 947–948.
Kozak LP, Anunciado-Koza R . UCP1: its involvement and utility in obesity. Int J Obes (Lond) 2008; 32 (Suppl 7): S32–S38.
Nedergaard J, Petrovic N, Lindgren EM, Jacobsson A, Cannon B . PPARgamma in the control of brown adipocyte differentiation. Biochim Biophys Acta 2005; 1740: 293–304.
Granneman JG, Li P, Zhu Z, Lu Y . Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab 2005; 289: E608–E616.
Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S . Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 2000; 279: C670–C681.
Ghorbani M, Himms-Hagen J . Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord 1997; 21: 465–475.
Kajimura S, Seale P, Spiegelman BM . Transcriptional control of brown fat development. Cell Metab 2010; 11: 257–262.
Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J . Chronic peroxisome proliferator-activated receptor {gamma} (PPAR{gamma}) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, ucp1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285: 7153–7164.
Murano I, Barbatelli G, Giordano A, Cinti S . Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat 2009; 214: 171–178.
Nedergaard J, Golozoubova V, Matthias A, Shabalina I, Ohba K, Ohlson K et al. Life without UCP1: mitochondrial, cellular and organismal characteristics of the UCP1-ablated mice. Biochem Soc Trans 2001; 29: 756–763.
Bouillaud F . UCP2, not a physiologically relevant uncoupler but a glucose sparing switch impacting ROS production and glucose sensing. Biochim Biophys Acta 2009; 1787: 377–383.
Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997; 387: 90–94.
Nedergaard J, Cannon B . The ‘novel’ ‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol 2003; 88: 65–84.
Nicholls DG, Locke RM . Thermogenic mechanisms in brown fat. Physiol Rev 1984; 64: 1–64.
Gonzalez-Barosso MMR E . The role of fatty acids in the activity of the uncoupling protein. Curr Chem Biol 2009; 3: 180–188.
Mozo J, Emre Y, Bouillaud F, Ricquier D, Criscuolo F . Thermoregulation: what role for UCPs in mammals and birds? Biosci Rep 2005; 25: 227–249.
Collins S, Cao W, Robidoux J . Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol 2004; 18: 2123–2131.
Rim JS, Kozak LP . Regulatory motifs for CREB-binding protein and Nfe2l2 transcription factors in the upstream enhancer of the mitochondrial uncoupling protein 1 gene. J Biol Chem 2002; 277: 34589–34600.
Watanabe M, Yamamoto T, Mori C, Okada N, Yamazaki N, Kajimoto K et al. Cold-induced changes in gene expression in brown adipose tissue: implications for the activation of thermogenesis. Biol Pharm Bull 2008; 31: 775–784.
Debevec D, Christian M, Morganstein D, Seth A, Herzog B, Parker M et al. Receptor interacting protein 140 regulates expression of uncoupling protein 1 in adipocytes through specific peroxisome proliferator activated receptor isoforms and estrogen-related receptor alpha. Mol Endocrinol 2007; 21: 1581–1592.
Bargmann W, von Hehn G, Lindner E . On the cells of the brown fatty tissue and their innervation]. Z Zellforsch Mikrosk Anat 1968; 85: 601–613.
Bartness TJ, Song CK . Innervation of brown adipose tissue and its role in thermogenesis. Can J Diabetes 2005; 29: 420–428.
Landsberg L, Saville ME, Young JB . Sympathoadrenal system and regulation of thermogenesis. Am J Physiol 1984; 247: E181–E189.
Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK et al. BetaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 2002; 297: 843–845.
Lowell BB, Bachman ES . Beta-adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem 2003; 278: 29385–29388.
Jimenez M, Leger B, Canola K, Lehr L, Arboit P, Seydoux J et al. Beta(1)/beta(2)/beta(3)-adrenoceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Lett 2002; 530: 37–40.
Richard D . Energy expenditure: a critical determinant of energy balance with key hypothalamic controls. Minerva Endocrinol 2007; 32: 173–183.
Berthoud HR, Morrison C . The brain, appetite, and obesity. Annu Rev Psychol 2008; 59: 55–92.
Grill HJ . Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 2006; 14 (Suppl 5): 216S–221S.
Cone RD . Studies on the physiological functions of the melanocortin system. Endocr Rev 2006; 27: 736–749.
Ellacott KL, Cone RD . The role of the central melanocortin system in the regulation of food intake and energy homeostasis: lessons from mouse models. Philos Trans R Soc Lond B Biol Sci 2006; 361: 1265–1274.
Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M . The MC4 receptor and control of appetite. Br J Pharmacol 2006; 149: 815–827.
Butler AA . The melanocortin system and energy balance. Peptides 2006; 27: 281–290.
Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD . A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA 2000; 97: 12339–12344.
Glavas MM, Joachim SE, Draper SJ, Smith MS, Grove KL . Melanocortinergic activation by melanotan II inhibits feeding and increases uncoupling protein 1 messenger ribonucleic acid in the developing rat. Endocrinology 2007; 148: 3279–3287.
Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ . Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 2007; 148: 5339–5347.
Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ . Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 2005; 289: R1467–R1476.
Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD et al. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 2007; 148: 1550–1560.
Harrold JA, Williams G . Melanocortin-4 receptors, beta-MSH and leptin: key elements in the satiety pathway. Peptides 2006; 27: 365–371.
Oswal A, Yeo GS . The leptin melanocortin pathway and the control of body weight: lessons from human and murine genetics. Obes Rev 2007; 8: 293–306.
Ilnytska O, Argyropoulos G . The role of the Agouti-Related Protein in energy balance regulation. Cell Mol Life Sci 2008; 65: 2721–2731.
Zhang Y, Kilroy GE, Henagan TM, Prpic-Uhing V, Richards WG, Bannon AW et al. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J 2005; 19: 1482–1491.
Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB . Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 2005; 493: 63–71.
Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ . The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 2002; 110: 515–526.
Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 1998; 21: 1375–1385.
Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK . Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 2003; 457: 213–235.
Madden CJ, Morrison SF . Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2010; 298: R776–R783.
Nogueira MI, de Rezende BD, do Vale LE, Bittencourt JC . Afferent connections of the caudal raphe pallidus nucleus in rats: a study using the fluorescent retrograde tracers fluorogold and true-blue. Ann Anat 2000; 182: 35–45.
Fan W, Morrison SF, Cao WH, Yu P . Thermogenesis activated by central melanocortin signaling is dependent on neurons in the rostral raphe pallidus (rRPa) area. Brain Res 2007; 1179: 61–69.
Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL . Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 1999; 33: 542–547.
Harthoorn LF . Projection-dependent differentiation of melanin-concentrating hormone-containing neurons. Cell Mol Neurobiol 2007; 27: 49–55.
Llewellyn-Smith IJ, Martin CL, Marcus JN, Yanagisawa M, Minson JB, Scammell TE . Orexin-immunoreactive inputs to rat sympathetic preganglionic neurons. Neurosci Lett 2003; 351: 115–119.
Nedergaard J, Bengtsson T, Cannon B . Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293: E444–E452.
Enerback S . Human brown adipose tissue. Cell Metab 2010; 11: 248–252.
Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531.
Garcia CA, Van Nostrand D, Atkins F, Acio E, Butler C, Esposito G et al. Reduction of brown fat 2-deoxy-2-[F-18]fluoro-D-glucose uptake by controlling environmental temperature prior to positron emission tomography scan. Mol Imaging Biol 2006; 8: 24–29.
Kim S, Krynyckyi BR, Machac J, Kim CK . Temporal relation between temperature change and FDG uptake in brown adipose tissue. Eur J Nucl Med Mol Imaging 2008; 35: 984–989.
Nedergaard J, Cannon B . The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 2010; 11: 268–272.
Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass and glucose-uptake activity of 18F-FDG-detected BAT. J Clin Endocrinol Metab 2011; e-pub ahead of print 13 October 2010; doi:10.1210/jc.2010-0989.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
ACC has received lecture fees from Pfizer, grant support from GlaxoSmithKline, Pfizer, Philips, Merck and Co. and Amsterdam Molecular Therapeutics, and holds two patents related to this subject area. The remaining authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Richard, D., Carpentier, A., Doré, G. et al. Determinants of brown adipocyte development and thermogenesis. Int J Obes 34 (Suppl 2), S59–S66 (2010). https://doi.org/10.1038/ijo.2010.241
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.241
Keywords
This article is cited by
-
Bovine lactoferrin promotes energy expenditure via the cAMP-PKA signaling pathway in human reprogrammed brown adipocytes
BioMetals (2018)
-
Induction of thermogenic adipocytes: molecular targets and thermogenic small molecules
Experimental & Molecular Medicine (2017)
-
Optimizing the methodology for measuring supraclavicular skin temperature using infrared thermography; implications for measuring brown adipose tissue activity in humans
Scientific Reports (2017)
-
Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers
European Journal of Nutrition (2016)
-
Role of BMP7 in appetite regulation, adipogenesis, and energy expenditure
Endocrine (2015)