Abstract
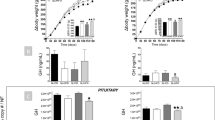
The pancreas is a major source of ghrelin in the perinatal period, whereas gastric production progressively increases after birth. Loss of function of the genes for ghrelin or for the constitutively activated growth hormone secretagogue receptor (GHSR) does not affect birth weight and early postnatal growth. However, ghrl−/− or ghsr−/− mice fed a high fat diet starting soon after weaning are resistant to diet-induced obesity, suggesting that ghrelin affects the maturation of the metabolic axes involved in energy balance. In addition, animal and human studies suggest that GHSR plays a physiological role in linear growth. In mice, absence of the GHSR gene is associated with lower insulin-like growth factor 1 concentrations and lower body mass in adult animals, independently of food intake. In humans, a mutation of the GHSR gene that impairs the constitutive activity of the receptor was found in two families with short stature. Administration of acylated ghrelin to rat pups directly does not affect weight gain. In contrast, administration of ghrelin to pregnant or lactating rats results in greater fetal weight and postnatal weight gain, respectively, suggesting that maternal ghrelin may stimulate perinatal growth. These data point toward a physiological role for ghrelin and GHSR in growth and/or in the maturation of hormonal systems involved in the regulation of energy balance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Zhu X, Cao Y, Voogd K, Steiner DF . On the processing of proghrelin to ghrelin. J Biol Chem 2006; 281: 38867–38870.
Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 2001; 86: 4753–4758.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K . Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999; 402: 656–660.
Sun Y, Wang P, Zheng H, Smith RG . Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 2004; 101: 4679–4684.
van der Lely AJ, Tschöp M, Heiman ML, Ghigo E . Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004; 25: 426–457.
Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW . High constitutive signaling of the ghrelin receptor—identification of a potent inverse agonist. Mol Endocrinol 2003; 17: 2201–2210.
Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 2004; 145: 234–242.
Chanoine JP, Wong AC . Ghrelin gene expression is markedly higher in fetal pancreas compared with fetal stomach: effect of maternal fasting. Endocrinology 2004; 145: 3813–3820.
Chanoine JP, Wong AC, Barrios V . Obestatin, acylated and total ghrelin concentrations in the perinatal rat pancreas. Horm Res 2006; 66: 81–88.
Hayashida T, Nakahara K, Mondal MS, Date Y, Nakazato M, Kojima M et al. Ghrelin in neonatal rats: distribution in stomach and its possible role. J Endocrinol 2002; 173: 239–245.
Walia P, Asadi A, Kieffer TJ, Johnson JD, Chanoine JP . Ontogeny of ghrelin, obestatin, preproghrelin, and prohormone convertases in rat pancreas and stomach. Pediatr Res 2008; e-pub ahead of print 3 September 2008.
Heller RS, Jenny M, Collombat P, Mansouri A, Tomasetto C, Madsen OD et al. Genetic determinants of pancreatic epsilon-cell development. Dev Biol 2005; 286: 217–224.
Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L . Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci USA 2004; 101: 2924–2929.
Farquhar J, Heiman M, Wong AC, Wach R, Chessex P, Chanoine JP . Elevated umbilical cord ghrelin concentrations in small for gestational age neonates. J Clin Endocrinol Metab 2003; 88: 4324–4327.
Wierup N, Svensson H, Mulder H, Sundler F . The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 2002; 107: 63–69.
Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA 2004; 101: 8227–8232.
Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO et al. Absence of ghrelin protects against early-onset obesity. J Clin Invest 2005; 115: 3573–3578.
Chanoine JP, Yeung LP, Wong AC . Umbilical cord ghrelin concentrations in Asian and Caucasian neonates. Horm Res 2003; 60: 116–120.
Pantel J, Legendre M, Cabrol S, Hilal L, Hajaji Y, Morisset S et al. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest 2006; 116: 760–768.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS . A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714–1719.
Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML . Circulating ghrelin levels are decreased in human obesity. Diabetes 2001; 50: 707–709.
Grove KL, Cowley MA . Is ghrelin a signal for the development of metabolic systems? J Clin Invest 2005; 115: 3393–3397.
Grove KL, Smith MS . Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav 2003; 79: 47–63.
Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 2005; 115: 3564–3572.
James RJ, Drewett RF, Cheetham TD . Low cord ghrelin levels in term infants are associated with slow weight gain over the first 3 months of life. J Clin Endocrinol Metab 2004; 89: 3847–3850.
Iniguez G, Ong K, Pena V, Avila A, Dunger D, Mericq V . Fasting and post-glucose ghrelin levels in SGA infants: relationships with size and weight gain at one year of age. J Clin Endocrinol Metab 2002; 87: 5830–5833.
Mackelvie KJ, Meneilly GS, Elahi D, Wong AC, Barr SI, Chanoine JP . Regulation of appetite in lean and obese adolescents after exercise: role of acylated and desacyl ghrelin. J Clin Endocrinol Metab 2007; 92: 648–654.
Stock S, Leichner P, Wong AC, Ghatei MA, Kieffer TJ, Bloom SR et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab 2005; 90: 2161–2168.
Soriano-Guillen L, Barrios V, Campos-Barros A, Argente J . Ghrelin levels in obesity and anorexia nervosa: effect of weight reduction or recuperation. J Pediatr 2004; 144: 36–42.
Bittel DC, Butler MG . Prader–Willi syndrome: clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med 2005; 7: 1–20.
Haqq AM, Farooqi IS, O'Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader–Willi syndrome. J Clin Endocrinol Metab 2003; 88: 174–178.
DelParigi A, Tschöp M, Heiman ML, Salbe AD, Vozarova B, Sell SM et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader–Willi syndrome. J Clin Endocrinol Metab 2002; 87: 5461–5464.
Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 2002; 8: 643–644.
De Waele K, Ishkanian SL, Bogarin R, Miranda CA, Ghatei MA, Stephen R et al. Long-acting octreotide treatment causes a sustained decrease in ghrelin concentrations but does not affect weight, behaviour and appetite in subjects with Prader Willi syndrome. Eur J Endocrinol 2008; 159: 381–388.
Nakahara K, Nakagawa M, Baba Y, Sato M, Toshinai K, Date Y et al. Maternal ghrelin plays an important role in rat fetal development during pregnancy. Endocrinology 2006; 147: 1333–1342.
Nakahara K, Hayashida T, Nakazato M, Kojima M, Hosoda H, Kangawa K et al. Effect of chronic treatments with ghrelin on milk secretion in lactating rats. Biochem Biophys Res Commun 2003; 303: 751–755.
Tham E, Liu J, Gaylinn B, Thorner M, Thompson D, Chanoine J-P . Acyl and des-acyl ghrelin concentrations during pregnancy and early postpartum in women with and without gestational diabetes. Can J Diabetes 2007; 31: 269 (OR 45).
Acknowledgements
This work was supported in part by Grant MOP 81291 of the Canadian Institutes of Health and Research (CIHR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
J-P Chanoine has received consulting fees from Novo Nordisk and Nestle as well as lecture fees from Roche. K De Waele and P Walia have declared no financial interests.
Rights and permissions
About this article
Cite this article
Chanoine, JP., De Waele, K. & Walia, P. Ghrelin and the growth hormone secretagogue receptor in growth and development. Int J Obes 33 (Suppl 1), S48–S52 (2009). https://doi.org/10.1038/ijo.2009.17
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.17
Keywords
This article is cited by
-
The maternal reduced uteroplacental perfusion model of preeclampsia induces sexually dimorphic metabolic responses in rat offspring
Biology of Sex Differences (2022)
-
Association of serum ghrelin with weight gain during pregnancy in overweight and normal women
Journal of Endocrinological Investigation (2019)
-
Association of cord blood des-acyl ghrelin with birth weight, and placental GHS-R1 receptor expression in SGA, AGA, and LGA newborns
Endocrine (2016)
-
Perinatal Oxidative Stress May Affect Fetal Ghrelin Levels in Humans
Scientific Reports (2015)
-
Regulation of NR4A by nutritional status, gender, postnatal development and hormonal deficiency
Scientific Reports (2014)