Abstract
Background:
Adipose tissue is a primary in vivo site of inflammation in obesity. Excess visceral adipose tissue (VAT), when compared to subcutaneous adipose tissue (SAT), imparts an increased risk of obesity-related comorbidities and mortality, and exhibits differences in inflammation. Defining depot-specific differences in inflammatory function may reveal underlying mechanisms of adipose-tissue-based inflammation.
Methods:
Stromovascular cell fractions (SVFs) from VAT and SAT from obese humans undergoing bariatric surgery were studied in an in vitro culture system with transcriptional profiling, flow cytometric phenotyping, enzyme-linked immunosorbent assay and intracellular cytokine staining.
Results:
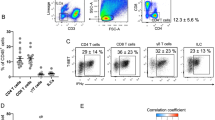
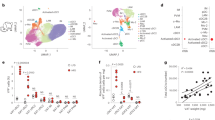
Transcriptional profiling of SVF revealed differences in inflammatory transcript levels in VAT relative to SAT, including elevated interferon-γ (IFN-γ) transcript levels. VAT demonstrated a broad leukocytosis relative to SAT that included macrophages, T cells and natural killer (NK) cells. IFN-γ induced a proinflammatory cytokine expression pattern in SVF and adipose tissue macrophages (ATM). NK cells, which constitutively expressed IFN-γ, were present at higher frequency in VAT relative to SAT. Both T and NK cells from SVF expressed IFN-γ on activation, which was associated with tumor necrosis factor-α expression in macrophages.
Conclusion:
These data suggest involvement of NK cells and IFN-γ in regulating ATM phenotype and function in human obesity and a potential mechanism for the adverse physiologic effects of VAT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005; 54: 2277–2286.
Lumeng CN, Bodzin JL, Saltiel AR . Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184.
Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2005; 116: 115–124.
Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N et al. Over-expression of MCP-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 2006; 281: 26602–26614.
Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (London) 2007; 31: 1420–1428.
Carey VJ, Walters EE, Colditz GA, Solomon CG, Willet WC, Rosner BA et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. Nurses’ Health Study. Am J Epidemiol 1997; 145: 614–619.
Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982; 54: 254–260.
Gómez-Ambrosi J, Catalán V, Diez-Caballero A, Martinez-Cruz LA, Gil MJ, García-Foncillas J et al. Gene expression profile of omental adipose tissue in human obesity. FASEB J 2004; 18: 215–217.
Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res 2004; 12: 1217–1222.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Roederer M . Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry 2001; 45: 194–205.
Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin-alpha are ligands for herpesvirus entry mediator. Immunity 1998; 8: 21–30.
Kim WJ, Kang YJ, Koh EM, Ahn KS, Cha HS, Lee WH . LIGHT is involved in the pathogenesis of rheumatoid arthritis by inducing the expression of pro-inflammatory cytokines and MMP-9 in macrophages. Immunology 2005; 114: 272–279.
Wang J, Lo JC, Foster A, Yu P, Chen HM, Wang Y et al. The regulation of T-cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest 2001; 108: 1771–1780.
Cohavy O, Zhou J, Granger SW, Ware CF, Targan SR . LIGHT expression by mucosal T cells may regulate IFN-g expression in the intestine. J Immunol 2004; 173: 251–258.
Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon BS et al. LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in-vivo tumor formation via gene transfer. J Clin Invest 1998; 102: 1142–1151.
Willis D, Moore AR, Frederick R, Willoughby DA . Haem oxygenase: a novel target for the modulation of the inflammatory response. Nat Med 1996; 2: 87–90.
Platt JL, Nath KA . Haem oxygenase: protective gene or Trojan horse. Nat Med 1998; 4: 1364–1365.
Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 2000; 6: 422–428.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A . Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23: 549–555.
Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD . GM-CSF and M-CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol 2007; 178: 5245–5252.
Makino M, Maeda Y, Fukutomi Y, Mukai T . Contribution of GM-CSF on the enhancement of the T cell-stimulating activity of macrophages. Microbes Infect 2007; 9: 70–77.
Ganguly D, Paul K, Bagchi J, Rakshit S, Mandal L, Bandyopadhyay G et al. Granulocyte–macrophage colony-stimulating factor drives monocytes to CD14loCD83+DCSIGN− interleukin-10-producing myeloid cells with differential effects on T-cell subsets. Immunology 2007; 121: 499–507.
Fried SK, Bunkin DA, Greenberg AS . Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J. Clin Endocrinol Metab 1998; 83: 847–850.
van Beek EA, Bakker AH, Kruyt PM, Hofker MH, Saris WH, Keijer J . Intra- and inter-individual variation in gene expression in human adipose tissue. Pflugers Arch 2007; 453: 851–861.
Hube F, Birgel M, Lee YM, Hauner H . Expression pattern of tumour necrosis factor receptors in subcutaneous and omental human adipose tissue: role of obesity and non-insulin-dependent diabetes mellitus. Eur J Clin Invest 1999; 29: 672–678.
Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 2008; 28: 1304–1310.
Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007; 115: 1029–1038.
Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 2008; 103: 467–476.
Permana PA, Menge C, Reaven PD . Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun 2006; 341: 507–514.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830.
Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol 2000; 164: 4105–4110.
Kosuge H, Suzuki J, Kakuta T, Haraguchi G, Koga N, Futamatsu H et al. Attenuation of graft arterial disease by manipulation of the LIGHT pathway. Arterioscler Thromb Vasc Biol 2004; 24: 1409–1415.
Scheu S, Alferink J, Pötzel T, Barchet W, Kalinke U, Pfeffer K . Targeted disruption of LIGHT causes defects in costimulatory T-cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med 2002; 195: 1613–1624.
Morel Y, Schiano de Collela JM, Harrop J, Deen KC, Homes SD, Wattam TA et al. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol 2000; 165: 4397–4404.
Fan Z, Yu P, Wang Y, Wang Y, Fu ML, Liu W et al. NK-cell activation by LIGHT triggers tumor-specific CD8+T-cell immunity to reject established tumors. Blood 2006; 107: 1342–1351.
Lee WH, Kim SH, Lee Y, Lee BB, Kwon B, Song H et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol 2001; 21: 2004–2010.
Grage-Griebenow E, Flad HD, Ernst M . Heterogeneity of human peripheral blood monocyte subsets. J Leukocyte Biol 2001; 69: 11–20.
Gordon S, Taylor PR . Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5: 953–964.
Tchoukalova YD, Koutsari C, Karpyak MV, Votruba SB, Wendland E, Jensen MD . Subcutaneous adipocyte size and body fat distribution. Am J Clin Nutr 2008; 87: 56–63.
Klimcakova E, Moro C, Mazzucotelli A, Lolmède K, Viguerie N, Galitzky J et al. Profiling of adipokines secreted from human subcutaneous adipose tissue in response to PPAR agonists. Biochem Biophys Res Commun 2007; 358: 897–902.
Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 2007; 56: 1517–1526.
Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 2008; 117: 806–815.
Darwich L, Coma G, Peña R, Bellido R, Blanco EJ, Este JA et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin IL-12 plus IL-18. Immunology 2009; 126: 386–393.
Acknowledgements
This work was supported by an American Surgical Association Foundation Fellowship Award (RWO), National Institutes of Health grants K08 DK074397 (RWO), K23 DK066165 (BAJ), R03 CA105959 (BAJ), AI054458 (MKS), an Oregon National Primate Research Center grant RR00163 (MKS) as well as the Oregon Clinical and Translational Research Institute, grant numbers UL1 RR024140 and TL1 RR024159 from the National Center for Research Resources, a component of the National Institutes of Health, and National Institutes of Health Roadmap for Medical Research (BRW and RWO). We thank Ms Nichelle Tran for expert administrative and graphic design assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O'Rourke, R., Metcalf, M., White, A. et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-γ in inflammation in human adipose tissue. Int J Obes 33, 978–990 (2009). https://doi.org/10.1038/ijo.2009.133
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.133
Keywords
This article is cited by
-
Association between predicted fat mass, predicted lean mass, predicted percent fat and type 2 diabetes mellitus in Japanese adults: a retrospective study
BMC Endocrine Disorders (2024)
-
A STAT5-Smad3 dyad regulates adipogenic plasticity of visceral adipose mesenchymal stromal cells during chronic inflammation
npj Regenerative Medicine (2022)
-
Natural killer cell-derived exosomal miR-1249-3p attenuates insulin resistance and inflammation in mouse models of type 2 diabetes
Signal Transduction and Targeted Therapy (2021)
-
DNA methylation and adiposity phenotypes: an epigenome-wide association study among adults in the Strong Heart Study
International Journal of Obesity (2020)
-
Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP)
Breast Cancer Research (2019)