Abstract
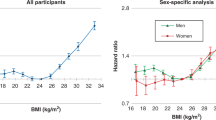
Obesity has been implicated in the aetiology of liver disease. However, to date, evidence is largely drawn from cross-sectional studies, where interpretation is hampered by reverse causality, and from studies on clinical populations that have limited generalisability. In this prospective cohort study, data on body mass index (BMI) and covariates were collected at baseline on 18 863 male government employees (aged 40–69 years). Respondents were then followed up for a maximum of 38 years of age. Mortality surveillance gave rise to 13 129 deaths, 122 of which were due to liver disease (57 cancers; 65 non-cancers). In age-adjusted analyses, BMI was positively related to total liver disease mortality (hazards ratio per 1 s.d. increase in BMI; 95% confidence interval (CI): 1.36; 1.14, 1.62) in a graded fashion across the weight categories (P-value for trend: 0.01). The magnitude of this association was somewhat stronger for non-cancer liver disease deaths (1.47; 1.16, 1.86) than for cancer liver disease deaths (1.25; 0.96, 1.62). Excluding deaths in the first 10 years of follow-up somewhat strengthened the BMI—non-cancer liver disease association. Adjustment for socioeconomic position, other candidate confounders and mediating factors led to the modest attenuation of these associations. Further investigation in prospective cohort studies with more detailed data on liver disease, for instance using biochemical tests of liver function or hepatic ultrasonography, is warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Leyland AH, Dundas R, McLoone P, Boddy F . Inequalities in mortality in Scotland 1981–2001 (Occasional paper No. 16). UK MRC Social and Public Health Sciences Unit: Glasgow, 2007.
Noble B . Deaths associated with the use of alcohol, drugs, and volatile substances. Popul Trends 1994; 76: 7–16.
Fisher NC, Hanson J, Phillips A, Rao JN, Swarbrick ET . Mortality from liver disease in the West Midlands, 1993–2000: observational study. BMJ 2002; 325: 312–313.
Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40: 1387–1395.
Lelbach WK . Epidemiology of alcoholic liver disease. In: Popper H, Schaffner F (eds). Progress in liver diseases. Grune and Stratton: Philadelphia, 1976.
Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL . Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 1998; 22: 39–47.
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM . Prevalence of Overweight and Obesity in the United States, 1999–2004. JAMA 2006; 295: 1549–1555.
Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology 2002; 35: 635–638.
Angulo P, Keach JC, Batts KP, Lindor KD . Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999; 30: 1356–1362.
Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC . Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25: 108–111.
Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology 1999; 29: 1215–1219.
Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G . Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 2001; 33: 1358–1364.
Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 2000; 132: 112–117.
Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol 2005; 43: 508–514.
Ioannou GN, Weiss NS, Kowdley KV, Dominitz JA . Is obesity a risk factor for cirrhosis-related death or hospitalization? A population-based cohort study. Gastroenterology 2003; 125: 1053–1059.
Reid DD, Hamilton PJS, McCartney P, Rose G, Jarrett RJ, Keen H et al. Cardiorespiratory disease and diabetes among middle-aged male civil servants. Lancet 1974; i: 469–473.
World Health Organisation. Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee. WHO Tech. Rep. Ser. Geneva: WHO, 1995.
Durrleman S, Simon R . Flexible regression models with cubic splines. Stat Med 1989; 8: 551–561.
Larsson SC, Wolk A . Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer 2007; 97: 1005–1008.
Wanless IR, Lentz JS . Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 1990; 12: 1106–1110.
Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, Smith GD . Obesity and overweight in relation to organ-specific cancer mortality in London (UK): findings from the original Whitehall study. Int J Obes (Lond) 2005; 29: 1267–1274.
Acknowledgements
The original screening of the Whitehall study was funded by the Department of Health and Social Security and the Tobacco Research Council. David Batty is a Wellcome Trust Fellow; Michael Marmot is a UK Medical Research Council Research Professor. Martin Shipley is supported by the British Heart Foundation and Mika Kivimaki by the Academy of Finland.Rachel Huxley and David Batty generated the idea for the present manuscript, and jointly wrote a first draft around analyses conducted by Martin Shipley. The remaining authors commented extensively on drafts of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Batty, G., Shipley, M., Kivimaki, M. et al. Obesity and overweight in relation to liver disease mortality in men: 38 year follow-up of the original Whitehall study. Int J Obes 32, 1741–1744 (2008). https://doi.org/10.1038/ijo.2008.162
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.162
Keywords
This article is cited by
-
Risk of chronic liver disease in post-menopausal women due to body mass index, alcohol and their interaction: a prospective nested cohort study within the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS)
BMC Public Health (2017)
-
Obesity, overweight and liver disease in the Midspan prospective cohort studies
International Journal of Obesity (2010)