Abstract
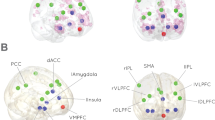
Three decades of research have investigated brain processing of visual sexual stimuli with neuroimaging methods. These researchers have found that sexual arousal stimuli elicit activity in a broad neural network of cortical and subcortical brain areas that are known to be associated with cognitive, emotional, motivational and physiological components. However, it is not completely understood how these neural systems integrate and modulated incoming information. Therefore, we identify cerebral areas whose activations were correlated with sexual arousal using event-related functional magnetic resonance imaging and used the dynamic causal modeling method for searching the effective connectivity about the sexual arousal processing network. Thirteen heterosexual males were scanned while they passively viewed alternating short trials of erotic and neutral pictures on a monitor. We created a subset of seven models based on our results and previous studies and selected a dominant connectivity model. Consequently, we suggest a dynamic causal model of the brain processes mediating the cognitive, emotional, motivational and physiological factors of human male sexual arousal. These findings are significant implications for the neuropsychology of male sexuality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain 2002; 125: 1014–1023.
Beauregard M, Levesque J, Bourgouin P . Neural correlates of conscious self-regulation of emotion. J Neurosci 2001; 21: RC165.
Redouté J, Stoléru S, Grégoire MC, Costes N, Cinotti L, Lavenne F et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp 2000; 11: 162–177.
Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V . Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci Biobehav Rev 2012; 36: 1481–1509.
Friston KJ, Harrison L, Penny W . Dynamic causal modelling. Neuroimage 2003; 19: 1273–1302.
Desmond JE, Glover GH . Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods 2002; 118: 115–128.
Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings. The Center for Research in Psychophysiology, University of Florida: Gainesville, FL, USA, 1999.
Walter M, Bermpohl F, Mouras H, Schiltz K, Tempelmann C, Rotte M et al. Distinguishing specific sexual and general emotional effects in fMRI—Subcortical and cortical arousal during erotic picture viewing. Neuroimage 2008; 40: 1482–1494.
Harrison L, Penny WD, Friston K . Multivariate autoregressive modeling of fMRI time series. Neuroimage 2003; 19: 1477–1491.
Stephan KE, Weiskopf N, Drysdale PM, Robinson PA, Friston KJ . Comparing hemodynamic models with DCM. Neuroimage 2007; 38: 387–401.
Hamann S, Herman RA, Nolan CL, Wallen K . Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci 2004; 7: 411–416.
Holstege G, Georgiadis JR, Paans AM, Meiners LC, van der Graaf FH, Reinders AS . Brain activation during human male ejaculation. J Neurosci 2003; 23: 9185–9193.
Mouras H, Stoléru S, Bittoun J, Glutron D, Pélégrini-Issac M, Paradis A-L et al. Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. Neuroimage 2003; 20: 855–869.
Moulier V, Mouras H, Pélégrini-Issac M, Glutron D, Rouxel R, Grandjean B et al. Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. Neuroimage 2006; 33: 689–699.
Treue S, Trujillo JCM . Feature-based attention influences motion processing gain in macaque visual cortex. Nature 1999; 399: 575–579.
Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage 2005; 26: 1086–1096.
Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC . Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 2001; 32: 537–551.
O'Doherty J, Critchley H, Deichmann R, Dolan RJ . Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci 2003; 23: 7931–7939.
Burns JM, Swerdlow RH . Right orbitofrontal tumor with pedophilia symptom and constructional apraxia sign. Arch Neurol 2003; 60: 437–440.
Acknowledgements
This research was supported by Individual Basic Science & Engineering Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education(NRF-2015R1D1A1A01059095).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Seok, JW., Park, MS. & Sohn, JH. Neural pathways in processing of sexual arousal: a dynamic causal modeling study. Int J Impot Res 28, 184–188 (2016). https://doi.org/10.1038/ijir.2016.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijir.2016.27