Abstract
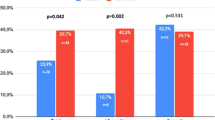
Despite the initial enthusiasm, the significant number of patients in whom sildenafil is contraindicated or ineffective is a major challenge to all urologists. Our aim was to determine the safety and efficacy of adjunctive atorvastatin in restoring normal erectile function in hypercholesterolemic (low-density lipoprotein (LDL) cholesterol >120 mg per 100 ml) sildenafil nonresponders. The study comprised 131 men with ED not responding to sildenafil citrate. They were randomized either to 40 mg atorvastatin daily (n=66, group 1) or matching placebo (n=65, group 2) for 12 weeks while they were taking on-demand 100 mg sildenafil. Erectile function was subjectively assessed using the 5-item version of the International Index of Erectile Function (IIEF-5) questionnaire and response to the global efficacy question (GEQ). Serum biochemical and lipid profile (total cholesterol, triglycerides, LDL cholesterol and high-density lipoprotein cholesterol) analyses were performed at baseline and repeated at post-treatment weeks 6 and 12. Compared with the placebo group (59 patients, mean age±s.d. 61.9±6.1, mean years ED 3.9±1.8), the atorvastatin group (59 patients, mean age±s.d. 63.9±6.9, mean years ED 3.7±1.6) had significantly greater improvements in all IIEF-5 questions (P=0.01) and GEQ (P=0.001). Subgroup analyses did reveal trends in the atorvastatin group to indicate that a change in the IIEF-5 score is affected by age, severity of ED and baseline serum levels of LDL. Patients with moderate (r=0.28, P=0.01) and severe (r=0.20, P=0.01) ED had better positive response rates to adjunctive atorvastatin than patients with mild to moderate ED. None of the patients taking atorvastatin achieved a response of 5 to the IIEF-5 questions and none of the patients regained normal erectile function as defined by the IIEF-5 score >21. Subjects experienced a statistically significant but modest improvement in erectile function. Further investigation is needed to test the usefulness of long-term atorvastatin administration to restore erectile function in sildenafil nonresponders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Laumann EO, Paik A, Rosen RC . Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999; 281: 537–544.
Safarinejad MR . Prevalence and risk factors for erectile dysfunction in a population-based study in Iran. Int J Impot Res 2003; 15: 246–252.
Aytaç IA, McKinlay JB, Krane RJ . The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999; 84: 50–56.
McKinlay JB . The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res 2000; 12 (Suppl 4): S6–11.
McMahon CG, Samali R, Johnson H . Efficacy, safety and patient acceptance of sildenafil citrate as treatment for erectile dysfunction. J Urol 2000; 164: 1192–1196.
Brisson TE, Broderick GA, Thiel DD, Heckman MG, Pinkstaff DM . Vardenafil rescue rates of sildenafil nonresponders: objective assessment of 327 patients with erectile dysfunction. Urology 2006; 68: 397–401.
Seftel AD . Challenges in oral therapy for erectile dysfunction. J Androl 2002; 23: 729–736.
Barada J . Successful salvage of sildenafil (Viagra) failures: benefits of patient education and rechallenge with sildenafil. Int J Impot Res 2001; 13 (Suppl 4): S49.
Jiann BP, Yu CC, Su CC, Huang JK . Rechallenge prior sildenafil nonresponders. Int J Impot Res 2004; 16: 64–68.
Rendell MS, Rajfer J, Wicker PA, Smith MD . Sildenafil for treatment of erectile dysfunction in men with diabetes. JAMA 1999; 281: 421–426.
Safarinejad MR . Oral sildenafil in the treatment of erectile dysfunction in diabetic men: a randomized double-blind and placebo-controlled study. J Diabetes Complications 2004; 18: 205–210.
Guay AT, Perez JB, Jacobson J, Newton RA . Efficacy and safety of sildenafil citrate for treatment of erectile dysfunction in a population with associated organic risk factors. J Androl 2001; 22: 793–797.
Safarinejad MR . Evaluation of the safety and efficacy of sildenafil citrate for erectile dysfunction in men with multiple sclerosis: a double-blind, placebo controlled, randomized study. J Urol 2009; 181: 252–258.
Safarinejad MR, Kolahi AA, Ghaedi G . Safety and efficacy of sildenafil citrate in treating erectile dysfunction in patients with combat-related post-traumatic stress disorder: a double-blind, randomized and placebo-controlled study. BJU Int 2009; 104: 376–383.
Safarinejad MR . Salvage of sildenafil failures with cabergoline: a randomized, double-blind, placebo-controlled study. Int J Impot Res 2006; 18: 550–558.
Safarinejad MR, Hosseini SY . Salvage of sildenafil failures with bremelanotide: a randomized, double-blind, placebo controlled study. J Urol 2008; 179: 1066–1071.
Doğru MT, Başar MM, Simşek A, Yuvanç E, Güneri M, Ebinç H et al. Effects of statin treatment on serum sex steroids levels and autonomic and erectile function. Urology 2008; 71: 703–707.
Kaiser DR, Billups K, Mason C, Wetterling R, Lundberg JL, Bank AJ . Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol 2004; 43: 179–184.
Kugiyama K, Ohgushi M, Sugiyama S, Murohara T, Fukunaga K, Miyamoto E et al. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Circ Res 1992; 71: 1422–1428.
Chang ST, Chu CM, Hsu JT, Lin PC, Shee JJ . Surveillance of cardiovascular risk factors for outpatients in different erectile dysfunction severity. Int J Impot Res 2009; 21: 116–121.
Eaton CB, Liu YL, Mittleman MA, Miner M, Glasser DB, Rimm EB . A retrospective study of the relationship between biomarkers of atherosclerosis and erectile dysfunction in 988 men. Int J Impot Res 2007; 19: 218–225.
Schouten BW, Bohnen AM, Bosch JL, Bernsen RM, Deckers JW, Dohle GR et al. Erectile dysfunction prospectively associated with cardiovascular disease in the Dutch general population: results from the Krimpen Study. Int J Impot Res 2008; 20: 92–99.
McFarlane SI, Muniyappa R, Francisco R, Sowers JR . Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab 2002; 87: 1451–1458.
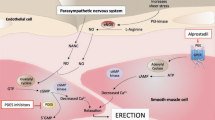
Castro MM, Rizzi E, Rascado RR, Nagassaki S, Bendhack LM, Tanus-Santos JE . Atorvastatin enhances sildenafil-induced vasodilation through nitric oxide-mediated mechanisms. Eur J Pharmacol 2004; 498: 189–194.
Morelli A, Chavalmane AK, Filippi S, Fibbi B, Silvestrini E, Sarchielli E et al. Atorvastatin ameliorates sildenafil-induced penile erections in experimental diabetes by inhibiting diabetes-induced RhoA/Rho-kinase signaling hyperactivation. J Sex Med 2009; 6: 91–106.
Hong SK, Han BK, Jeong SJ, Byun SS, Lee SE . Effect of statin therapy on early return of potency after nerve sparing radical retropubic prostatectomy. J Urol 2007; 178: 613–616.
Saltzman EA, Guay AT, Jacobson J . Improvement in erectile function in men with organic erectile dysfunction by correction of elevated cholesterol levels: a clinical observation. J Urol 2004; 172: 255–258.
Herrmann HC, Levine LA, Macaluso Jr J, Walsh M, Bradbury D, Schwartz S et al. Can atorvastatin improve the response to sildenafil in men with erectile dysfunction not initially responsive to sildenafil? Hypothesis and pilot trial results. J Sex Med 2006; 3: 303–308.
NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993; 270: 83–90.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A . The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile function. Urology 1997; 49: 822–830.
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM . Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999; 11: 319–326.
Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S . Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: a novel vasculoprotective function of statins. Circulation 2004; 110: 856–861.
Bank AJ, Kelly AS, Kaiser DR, Crawford WW, Waxman B, Schow DA et al. The effects of quinapril and atorvastatin on the responsiveness to sildenafil in men with erectile dysfunction. Vasc Med 2006; 11: 251–257.
Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB . Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994; 151: 54–61.
Puddu P, Puddu GM, Muscari A . HMG-CoA reductase inhibitors: is the endothelium the main target? Cardiology 2001; 95: 9–13.
Carvajal A, Macias D, Sáinz M, Ortega S, Martín Arias LH, Velasco A et al. HMG CoA reductase inhibitors and impotence: two case series from the Spanish and French drug monitoring systems. Drug Saf 2006; 29: 143–149.
Jackson G . Simvastatin and impotence. Br Med J 1997; 351: 31.
Boyd IW . Comment: HMG-CoA reductase inhibitor-induced impotence. Ann Pharmacother 1996; 30: 1199.
Solomon H, Samarasinghe YP, Feher MD, Man J, Rivas-Toro H, Lumb PJ et al. Erectile dysfunction and statin treatment in high cardiovascular risk patients. Int J Clin Pract 2006; 60: 141–145.
Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest 1990; 86: 228–234.
Acharya UR, Kannathal N, Sing OW, Ping LY, Chua T . Heart rate analysis in normal subjects of various age group. Biomed Eng Online 2004; 3: 24.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dadkhah, F., Safarinejad, M., Asgari, M. et al. Atorvastatin improves the response to sildenafil in hypercholesterolemic men with erectile dysfunction not initially responsive to sildenafil. Int J Impot Res 22, 51–60 (2010). https://doi.org/10.1038/ijir.2009.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijir.2009.48
Keywords
This article is cited by
-
Interactions between erectile dysfunction, cardiovascular disease and cardiovascular drugs
Nature Reviews Cardiology (2022)
-
Two Birds with One Stone: Regular Use of PDE5 Inhibitors for Treating Male Patients with Erectile Dysfunction and Cardiovascular Diseases
Cardiovascular Drugs and Therapy (2019)
-
Non-invasive Management Options for Erectile Dysfunction When a Phosphodiesterase Type 5 Inhibitor Fails
Drugs & Aging (2018)
-
A review of the positive and negative effects of cardiovascular drugs on sexual function: a proposed table for use in clinical practice
Netherlands Heart Journal (2014)