Abstract
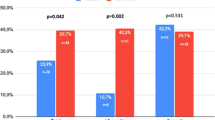
Phosphodiesterase type 5 inhibitors (PDE5Is) are the first-line therapeutic option for erectile dysfunction (ED), while second-line therapy includes the alprostadil. Due to the different pharmacodynamic mechanism of PDE5Is and alprostadil, a synergistic action is conceivable when they are administered in combination. The aim of present study was to evaluate the efficacy and safety of combination therapy with PDE5I and topical alprostadil in patients with ED non-responders to PDE5I alone. We designed a prospective, two-arm, open-label, non-randomized study. Patients over 18 years old, with a stable sexual relationship for at least 6 months, and ED non-responders to PDE5I monotherapy were included in the study. At baseline the variables assessed were 5-item version of the International Index of Erectile Function (IIEF-5), and Sexual Encounter Profile Questions 2 and 3 (SEP-2 and SEP-3). In addition, all subjects underwent penile dynamic duplex ultrasonography. All patients were assigned to the monotherapy group (Group A) or combination therapy group (Group B) based on their preference. Topical alprostadil 300 μg/100 mg (Virirec®) was the treatment assigned to Group A, while the combination therapy with the last PDE5I taken (at the maximum recommended dose) plus topical alprostadil 300 μg/100 mg (Virirec®) was assigned to Group B. After 3 months from assignment to groups were evaluated IIEF-5, SEP-2 and SEP-3 regarding the last sexual intercourse, and Global Assessment Questionnaire-Questions 1 and 2 (GAQ-1 and GAQ-2). All adverse events (AEs) that occurred during the study period were recorded. A total of 170 patients were included in the study (72 in Group A and 98 in Group B). Fifty-two patients were previously treated with sildenafil 100 mg (30.6%), 6 with vardenafil 20 mg (3.5%), 56 with tadalafil 20 mg (32.9%), and 56 with avanafil 200 mg (32.9%). No significant differences among the study groups were found at baseline (p > 0.05). The mean IIEF-5 score increased significantly in Group B after treatment compared to baseline (12.4 ± 3.4 vs. 17.1 ± 4.5; p < 0.001), conversely patients in Group A showed no significant increase (12.2 ± 2.5 vs. 12.7 ± 3.1; p = 0.148). The number of affirmative responses to SEP-2 was significantly higher after treatment compared to baseline only in Group B (57 vs. 78; p < 0.001). The number of affirmative responses to SEP-3 was significantly higher after treatment compared to baseline in both groups (p < 0.001). The number of affirmative responses to GAQ-Q1 and GAQ-Q2 was significantly higher in Group B compared to Group A (p < 0.001). A total of 59 (34.7%) patients experienced AEs. They were mild, self-limited, and did not cause discontinuation of treatment. No episode of priapism was recorded. No statistically significant difference was recorded between the AEs of the two groups, except for facial flushing that was reported only in Group B (p = 0.021). The combination therapy with topical alprostadil and PDE5I seems to be more effective than topical alprostadil alone without worsening the safety of the treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Salonia A, Bettocchi C, Carvalho J, Corona G, Jones TH, Kadioglu A, et al. Members of the EAU guidelines on sexual and reproductive health panel. Presented at the EAU Annual Congress Amsterdam 2020. 978-94-92671-07-3. Arnhem, The Netherlands: EAU Guidelines Office.
Cuzin B. Alprostadil cream in the treatment of erectile dysfunction: clinical evidence and experience. Ther Adv Urol. 2016;8:249–56.
Eardley I. The incidence, prevalence, and natural history of erectile dysfunction. Sex Med Rev. 2013;1:3–16.
Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61.
Wagner G, Fugl-Meyer KS, Fugl-Meyer AR. Impact of erectile dysfunction on quality of life: patient and partner perspectives. Int J Impot Res. 2000;12:S144–S6.
Latini DM, Penson DF, Lubeck DP, Wallace KL, Henning JM, Lue TF. Longitudinal differences in disease specific quality of life in men with erectile dysfunction: results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction study. J Urol. 2003;169:1437–42.
Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–13.
Munk NE, Knudsen JS, Comerma-Steffensen S, Simonsen U. Systematic review of oral combination therapy for erectile dysfunction when phosphodiesterase type 5 inhibitor monotherapy fails. Sex Med Rev. 2019;7:430–41.
Moncada I, Cuzin B. Clinical efficacy and safety of Vitaros©/Virirec© (Alprostadil cream) for the treatment of erectile dysfunction. Urologia. 2015;82:84–92.
Kim NN, Huang Y, Moreland RB, Kwak SS, Goldstein I, Traish A. Cross-regulation of intracellular cGMP and cAMP in cultured human corpus cavernosum smooth muscle cells. Mol Cell Biol Res Commun. 2000;4:10–4.
Moncada I, Martinez-Salamanca J, Ruiz-Castañe E, Romero J. Combination therapy for erectile dysfunction involving a PDE5 inhibitor and alprostadil. Int J Impot Res. 2018;30:203–8.
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–26.
Aversa A, Sarteschi LM. The role of penile color-duplex ultrasound for the evaluation of erectile dysfunction. J Sex Med. 2007;4:1437–47.
Golijanin D, Singer E, Davis R, Bhatt S, Seftel A, Dogra V. Doppler evaluation of erectile dysfunction—part 1. Int J Impot Res. 2007;19:37–42.
Carvalheira AA, Pereira NM, Maroco J, Forjaz V. Dropout in the treatment of erectile dysfunction with PDE5: a study on predictors and a qualitative analysis of reasons for discontinuation. J Sex Med. 2012;9:2361–9.
Cai T, Palumbo F, Liguori G, Mondaini N, Scroppo FI, Di Trapani D, et al. The intra-meatal application of alprostadil cream (Vitaros®) improves drug efficacy and patient’s satisfaction: results from a randomized, two-administration route, cross-over clinical trial. Int J Impot Res. 2019;31:119–25.
Eardley I, Donatucci C, Corbin J, El-Meliegy A, Hatzimouratidis K, McVary K, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010;7:524–40.
Hatzimouratidis K, Hatzichristou D. Phosphodiesterase type 5 inhibitors: the day after. Eur Urol. 2007;51:75–88.
Cheng E. Real-life safety and efficacy of vardenafil in the treatment of erectile dysfunction-results from 30,010 U.S. patients. J Sex Med. 2007;4:432–9.
Fisher WA, Eardley I, McCabe M, Sand M. Erectile dysfunction (ED) is a shared sexual concern of couples II: association of female partner characteristics with male partner ED treatment seeking and phosphodiesterase type 5 inhibitor utilization. J Sex Med. 2009;6:3111–24.
McMahon CN, Smith CJ, Shabsigh R. Treating erectile dysfunction when PDE5 inhibitors fail. BMJ. 2006;332:589–92.
Atiemo HO, Szostak MJ, Sklar GN. Salvage of sildenafil failures referred from primary care physicians. J Urol. 2003;170:2356–8.
Romero Otero J, García Gómez B, Medina Polo J, Jiménez Alcaide E, García Cruz E, Sallent Font A, et al. Evaluation of current errors within the administration of phosphodiesterase-5 inhibitors after more than 10 years of use. Urology. 2014;83:1334–8.
Park MG, Yeo JK, Cho DY, Kim JW, Kim JW, Oh MM, et al. The efficacy of combination treatment with injectable testosterone undecanoate and daily tadalafil for erectile dysfunction with testosterone deficiency syndrome. J Sex Med. 2015;12:966–74.
Aversa A, Francomano D, Lenzi A. Does testosterone supplementation increase PDE5-inhibitor responses in difficult-to-treat erectile dysfunction patients? Expert Opin Pharmacother. 2015;16:625–8.
Raina R, Agarwal A, Allamaneni SS, Lakin MM, Zippe CD. Sildenafil citrate and vacuum constriction device combination enhances sexual satisfaction in erectile dysfunction after radical prostatectomy. Urology. 2005;65:360–4.
Chen J, Sofer M, Kaver I, Matzkin H, Greenstein A. Concomitant use of sildenafil and a vacuum entrapment device for the treatment of erectile dysfunction. J Urol. 2004;171:292–5.
Canguven O, Bailen J, Fredriksson W, Bock D, Burnett AL. Combination of vacuum erection device and PDE5 inhibitors as salvage therapy in PDE5 inhibitor nonresponders with erectile dysfunction. J Sex Med. 2009;6:2561–7.
El Taieb M, Hegazy E, Ibrahim A. Daily oral l-arginine plus tadalafil in diabetic patients with erectile dysfunction: a double-blinded, randomized, controlled clinical trial. J Sex Med. 2019;16:1390–7.
Gentile V, Vicini P, Prigiotti G, Koverech A, Di, Silverio F. Preliminary observations on the use of propionyl-L-carnitine in combination with sildenafil in patients with erectile dysfunction and diabetes. Curr Med Res Opin. 2004;20:1377–84.
Cavallini G, Modenini F, Vitali G, Koverech A. Acetyl-L-carnitine plus propionyl-L-carnitine improve efficacy of sildenafil in treatment of erectile dysfunction after bilateral nerve-sparing radical retropubic prostatectomy. Urology. 2005;66:1080–5.
McMahon CG, Samali R, Johnson H. Treatment of intracorporeal injection nonresponse with sildenafil alone or in combination with triple agent intracorporeal injection therapy. J Urol. 1999;162:1992–8.
Nandipati K, Raina R, Agarwal A, Zippe CD. Early combination therapy: intracavernosal injections and sildenafil following radical prostatectomy increases sexual activity and the return of natural erections. Int J Impot Res. 2006;18:446–51.
Mydlo JH, Volpe MA, Macchia RJ. Initial results utilizing combination therapy for patients with a suboptimal response to either alprostadil or sildenafil monotherapy. Eur Urol. 2000;38:30–4.
Nehra A, Blute ML, Barrett DM, Moreland RB. Rationale for combination therapy of intraurethral prostaglandin E(1) and sildenafil in the salvage of erectile dysfunction patients desiring noninvasive therapy. Int J Impot Res. 2002;14:S38–42.
Raina R, Nandipati KC, Agarwal A, Mansour D, Kaelber DC, Zippe CD. Combination therapy: medicated urethral system for erection enhances sexual satisfaction in sildenafil citrate failure following nerve-sparing radical prostatectomy. J Androl. 2005;26:757–60.
John H, Lehmann K, Hauri D. Intraurethral prostaglandin improves quality of vacuum erection therapy. Eur Urol. 1996;29:224–6.
Labairu-Huerta L, Padilla-Fernández B, Arrondo-Arrondo JL, Valverde-Martínez LS, Martín-Rodríguez A, Silva-Abuín JM, et al. PDE-5 inhibitors in monotherapy versus combination therapy in a sample of 1200 patients with erectile dysfunction. Arch Ital Urol Androl. 2015;87:204–9.
Mantovani F. Alprostadil plus Vacuum (VITARUM) in severe erectile dysfunction (ED). Arch Ital Urol Androl. 2017;89:146–7. 30
Andersson KE. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharm Rev. 2011;63:811–59.
Anaissie J, Hellstrom WJ. Clinical use of alprostadil topical cream in patients with erectile dysfunction: a review. Res Rep Urol. 2016;8:123–31.
Arcaniolo D, Bellastella G, Manfredi C, Terribile M, Giordano DR, Quattrone C, et al. Is topical alprostadil an usable and reliable alternative to intracavernous injection for penile dynamic duplex ultrasonography? Andrologia. 2020;52:e13480.
Becher E. Topical alprostadil cream for the treatment of erectile dysfunction. Expert Opin Pharmacother. 2004;5:623–32.
Goldstein I, Payton TR, Schechter PJ. A double-blind, placebo-controlled, efficacy and safety study of topical gel formulation of 1% alprostadil (Topiglan) for the in-office treatment of erectile dysfunction. Urology. 2001;57:301–5.
Padma-Nathan H, Steidle C, Salem S, Tayse N, Yeager J, Harning R. The efficacy and safety of a topical alprostadil cream, Alprox-TD, for the treatment of erectile dysfunction: two phase 2 studies in mild-to-moderate and severe ED. Int J Impot Res. 2003;15:10–7.
Padma-Nathan H, Yeager JL. An integrated analysis of alprostadil topical cream for the treatment of erectile dysfunction in 1732 patients. Urology. 2006;68:386–91.
Rooney M, Pfister W, Mahoney M, Nelson M, Yeager J, Steidle C. Long-term, multicenter study of the safety and efficacy of topical alprostadil cream in male patients with erectile dysfunction. J Sex Med. 2009;6:520–34.
McVary KT. Clinical practice. Erectile Dysfunction. N Engl J Med. 2007;357:2472–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garrido-Abad, P., Senra-Bravo, I., Manfredi, C. et al. Combination therapy with topical alprostadil and phosphodiesterase-5 inhibitors after failure of oral therapy in patients with erectile dysfunction: a prospective, two-arm, open-label, non-randomized study. Int J Impot Res 34, 164–171 (2022). https://doi.org/10.1038/s41443-020-00400-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-020-00400-9