Abstract
This prospective cohort study compared measurements of maternal home blood pressure (HBP) with clinic blood pressure (CBP) before 20 weeks’ gestation to determine associations with the risk of delivering a lower birth weight infant. A total of 605 Japanese women were included. Exposures were initial CBP, made between 10 weeks 0 days and 19 weeks 0 days, and HBP for comparison made within 1 week of CBP. Outcome was infant’s birth weight, categorized and ranked as follows: ⩾3500 g, 3000–3499 g, 2500–2999 g and <2500 g. The proportional odds model with possible confounding factors was applied to compare the associations between CBP and HBP on infant birth weight. When both CBP and HBP were included simultaneously, the adjusted odds ratios (ORs) per 1 standard deviation (1s.d.) increase in clinic and home diastolic BP (DBP) were 1.06 (95% confidence interval (CI): 0.87–1.30) and 1.28 (95% CI: 1.04–1.58), respectively. The adjusted ORs per 1s.d. increase in clinic and home mean arterial pressure (MAP) were 1.02 (95% CI: 0.83–1.24) and 1.29 (95% CI: 1.04–1.59), respectively. Systolic BP measurement was not associated with infant birth weight. In conclusion, high maternal home DBP and MAP before 20 weeks’ gestation was associated with a higher risk of lower infant birth weight than clinic DBP and MAP. Therefore, in addition to CBP, it may be worth having pregnant women measure HBP to determine the risk of lower infant birth weight.
Similar content being viewed by others
Introduction
Birth weight reflects the quality of the intrauterine environment.1 There are continuous inverse relationships between an infant’s birth weight and future diseases such as hypertension, coronary artery disease, diabetes mellitus and stroke.2 An infant’s birth weight is affected by several factors during pregnancy including maternal physique, gestational weight gain, smoking status, complications and seasonality.3, 4, 5, 6, 7 One common maternal complication that affects birth weight is hypertensive disorder in pregnancy, including chronic hypertension and pregnancy-induced hypertension (PIH), which is defined as hypertension that occurs after 20 weeks’ gestation.8, 9 The prevalence of hypertensive disorder in pregnancy is about 5–10%, and the perinatal prognosis is poor owing to premature delivery, stillbirth or maternal death in addition to impaired fetal growth.8, 10, 11 Furthermore, women with pre-eclampsia have sustained high blood pressure (BP) after delivery.12 Therefore, BP monitoring throughout pregnancy is an essential part of perinatal care.
Conventionally, maternal BP measurements to diagnose hypertensive disorder in pregnancy have been made based on clinic BP (CBP) levels.9, 13 High maternal CBP and abnormal uterine artery doppler findings were related to abnormal level of soluble fms-like tyrosine kinase 1, soluble endoglin and placental growth factor.14 An inverse association between high maternal CBP levels during pregnancy and perinatal prognosis, in particular infant’s birth weight, has been reported.6
Several studies have also reported on the use of home BP (HBP) measurements.15, 16, 17, 18, 19 Results in both a general population and in patients with hypertension indicated that HBP was a better predictor of cardiovascular events than CBP.15, 16, 17, 18, 19 Although HBP during pregnancy was examined in previous studies,20, 21, 22, 23 no study has compared HBP with CBP during pregnancy to determine if these measurements are inversely related to infant birth weight. Because previous studies have shown that high CBP even before the clinical onset of PIH was related to PIH and small-for-gestational age (SGA) infants,13, 24 we focused on maternal BP before 20 weeks’ gestation in this study.
The aim of this study was to compare the association of HBP vs. CBP measurements before 20 weeks’ gestation on the risk of delivering infants with a lower birth weight.
Methods
Study design and participants
This study was a part of the Babies and their parents’ longitudinal Observation in Suzuki memorial Hospital on Intrauterine period (BOSHI) study—a prospective cohort study.23, 25, 26 The BOSHI study was conducted in Suzuki Memorial Hospital, an obstetrical and gynecological hospital in the Sendai city area, Miyagi prefecture in Japan. Details of the study are described elsewhere.23, 25, 26 Study protocols were approved by the institutional review board of Tohoku University School of Medicine and by the review board of Suzuki Memorial Hospital. All participants gave informed consent.
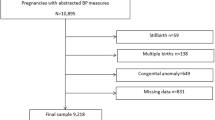
In the BOSHI study, 4069 women had a confirmed intrauterine pregnancy and booked delivery in this hospital between 16 October 2006, and 31 May 2010. Among them, 1473 women had the BOSHI study explained to them during this period, and 765 women consented to participate in this study before 20 weeks’ gestation. We excluded women treated with antihypertensive medication before 20 weeks’ gestation owing to chronic hypertension (6 women) and twin pregnancies (7 women). Fifteen women with chronic hypertension who did not use antihypertensive drugs before 20 weeks’ gestation were included in this study. Therefore, the remaining 752 women were included in this study.
HBP and CBP measurements
The methods for HBP23, 25, 26 and CBP26 measurement are described elsewhere.23, 25, 26 BP was measured using a semi-automatic device (HEM-747IC or HEM-7080IC for HBP and HEM705IT for CBP, respectively; Omron Healthcare, Kyoto, Japan) which is based on the cuff-oscillometric method and has a digital display system. These devices used cuffs and algorithms that were validated in pregnant women.27 The initial CBP was made between 10 weeks 0 days and 19 weeks 0 days, and the HBP measurement for comparison was made within 1 week of the CBP measurement. Therefore, the gestational age at HBP measurement ranged from 10 weeks 0 days to 19 weeks 6 days. CBP and HBP, which were each measured at least once, were evaluated. The mean of CBP values was used if more than 1 measurement was made. For HBP data, the mean of the first record on each day, which was made within 1 week of the CBP measurement, was used if more than one CBP measurement was used. Mean arterial pressure (MAP) of CBP and HBP was calculated as diastolic BP (DBP)+(systolic BP (SBP)−DBP)/3 in CBP and HBP, respectively.
Data collection and statistical analysis
Medical information about participants was collected from medical records, by questionnaires, and via surveys by midwives. The associations of maternal CBP and HBP with infant birth weight were examined using the proportional odds model with possible confounding factors, rather than multivariate linear regression model.28 Details of data collection and statistical analysis are described in the Supplementary Information.
Results
Maternal and neonatal characteristics of study participants
After 752 women were enrolled, 147 women were excluded. Reasons for exclusion from analysis and differences in maternal and neonatal characteristics between study participants and excluded women are described in the Supplementary Information. The remaining 605 women were analyzed. Table 1 shows baseline characteristics of participants who were analyzed. There were no women with a history of chronic kidney disease or women diagnosed with antiphospholipid syndrome before pregnancy. There was only one woman with systemic lupus erythematosus. Among study subjects, 73 women (12.1%) had only one CBP measurement. In addition, not all subjects measured HBP every day. The numbers of HBP measurements during a week were once (11.1%), twice (14.9%), 3 times (18.2%), 4 times (11.6%), 5 times (14.1%), 6 times (20.8%) and 7 times (9.4%).
Association between maternal CBP and infant birth weight
Table 2 shows results of the association between CBP and infant birth weight. In all analyses, the proportional odds assumption was not rejected statistically. No strong multicollinearity among explanatory variables was confirmed. Although the second quartile of clinic SBP was associated with lower categories of infant’s birth weight, no significant linear association between clinic SBP and infant birth weight existed (P for trend was 0.75). In contrast, higher clinic DBP was associated with lower infant’s birth weight (P for trend was 0.007). The adjusted odds ratio was 1.21 (95% confidence interval: 1.03–1.44) for each 1s.d. elevation of clinic DBP. Clinic MAP was not significantly associated with infant’s birth weight.
Association between maternal HBP and infant birth weight
The association between maternal HBP and infant birth weight is shown in Table 3. As with CBP, the proportional odds assumption was not rejected by the score test. There was no strong multicollinearity among explanatory variables.
In terms of home SBP, although the second quartile of home SBP was associated with lower categories of infant’s birth weight, no significant linear association existed (P for trend was 0.25). On the other hand, both home DBP and home MAP were at risk for delivering lower categories of infant’s birth weight (P for trend were 0.002 and 0.007, respectively). The adjusted odds ratios of home DBP and home MAP with each 1s.d. of increased BP were 1.33 (95% confidence interval, 1.11–1.58) and 1.30 (95% confidence interval, 1.09–1.55), respectively.
Comparison of CBP and HBP in terms of infant birth weight
CBP and HBP were included into the model as continuous variables simultaneously per 1s.d. change in the variables (Tables 4 and 5). In this analysis, the gestational week at CBP but not HBP measurement was included as an explanatory variable because gestational week at BP measurement had strong multicollinearity among CBP and HBP (that is, the results of analyses that included gestational week at HBP measurement, but not CBP, were comparable).
In terms of SBP, neither clinic SBP nor home SBP was significantly associated with infant birth weight (Table 4). Furthermore, the goodness-of-fit did not improve when clinic SBP and home SBP were included simultaneously compared with when each BP was included separately based on the likelihood ratio test (Tables 5, P=0.08 and 0.53, respectively). On the other hand, the statistical significance of home DBP remained, whereas that of clinic DBP diminished when clinic DBP and home DBP were included simultaneously (Table 4). The likelihood ratio test showed a significant improvement when home DBP was included in the analysis in addition to clinic DBP, whereas the inverse was not true (Tables 5, P=0.02 and 0.45, respectively). In addition, the statistical significance of home MAP also remained even after clinic MAP was included simultaneously (Table 4). As with DBP, the goodness-of-fit improved when home MAP was included in addition to clinic MAP, although the inverse was not true (Tables 5, P=0.02 and 0.60, respectively).
Discussion
These results indicate that maternal HBP before 20 weeks’ gestation was strongly related to the risk of delivering a lower birth weight infant compared with CBP. To the best of our knowledge, this is the first report that compared CBP and HBP measurements with the association with lower infant birth weight during pregnancy. Furthermore, this was also the first study that showed the association between high HBP during pregnancy and lower infant birth weight. Our results are similar to studies conducted in a general population or hypertensive subjects in whom HBP was a better predictor for prognosis than CBP, although the final outcomes examined in our study and these other studies differed.15, 16, 17, 18, 19 Several reasons for differences between CBP and HBP on lower birth weight should be considered. First, the white coat effect that occurs when CBP is measured might overestimate maternal hemodynamics. Second, the number and time of measurements among CBP and HBP were different. These discrepancies between CBP and HBP might have contributed to our findings.
Furthermore, we performed additional analyses to determine whether SBP or DBP was more strongly associated with lower infant birth weight (results are described in Supplementary Tables 2 and 3). As a result, both clinic and home DBP measurements were associated with a risk of lower birth weight infants, whereas SBP measurements were not. This finding is comparable with previous studies that were conducted in a general population and pregnant women. In a general population, the Framingham Heart Study showed that the impact of DBP on coronary heart disease was stronger than that of SBP in patients younger than 50 years.29 A recent study also showed that among subjects younger than 50 years, high DBP levels, as measured by 24-h ambulatory BP monitoring, were the predominant risk factor for coronary complications, whereas SBP levels were not.30 In the generation R study, increments in DBP during pregnancy were inversely associated with infant’s birth weight and SGA, whereas increments in SBP were not.6 In regard to the risk of low birth weight, the point estimate of the adjusted odds ratio of clinic DBP was higher than that of clinic SBP, although direct comparisons were not made.6 Furthermore, DBP at the first trimester was negatively associated with fetal crown to rump length, which was related to low birth weight and SGA; SBP was not significantly associated with these variables.31 One possible reason for this finding might be owing to the different mechanisms of BP increase between SBP and DBP. McEniery et al.32 reported that isolated systolic hypertension resulted from the increase in stroke volume and/or aortic stiffness in young people but not from the increase in peripheral vascular resistance. In contrast, DBP increases occur when peripheral vascular resistance increases.32 On the basis of different mechanisms of BP increase, Poon et al.13 also stated the importance of DBP during pregnancy. After conception, maternal vascular resistance decreases by trophoblast invasion and by remodeling of uterine spiral artery.33 This change is thought to be important to supply sufficient uteroplacental blood flow for ideal fetal growth.33 Thus high maternal vascular resistance may have a worse effect on fetal growth. Previous studies reported that high resistance of the uterine artery, measured at the first trimester using ultrasonography, was predictive of intrauterine growth restriction and SGA.34, 35 Maternal total vascular resistance (TVR) includes and correlates with uteroplacental resistance.36 Vasapollo et al. showed that maternal TVR at 20 to 22 weeks’ gestation was predictive of adverse outcomes including fetal growth restriction and PIH.37 Therefore, high maternal DBP may reflect high maternal TVR, in particular uteroplacental resistance, and subsequent fetal growth restriction and lower infant birth weight. MAP also reflects TVR.38 From our results, we estimate that, compared with CBP, home DBP and MAP may more strongly reflect TVR during pregnancy.
Furthermore, the same analyses were performed after 6 subjects who delivered infants with macrosomia (that is, birth weight ⩾4000 g) were excluded because several studies showed that macrosomia was a risk for type 2 diabetes and newborn complications.39, 40 After that, results were comparable (data not shown).
In this study, 73 women (12.1%) had one CBP measurement and 532 women (87.9%) had two CBP measurements. To examine the effect of difference in numbers of CBP measurements among study subjects, we reanalyzed the association between CBP and infant birth weight including numbers of CBP measurements as an explanatory variable in the proportional odds model. In this analysis, results were comparable (data not shown). Therefore, differences in numbers of CBP measurements among study subjects are unlikely to have had a strong effect on study findings.
There are several limitations to this study. First, this study was conducted in a single hospital and the rate of agreement to participate in this study was low (51.9%). Therefore, selection bias cannot be ruled out. However, the external validity of this study may be acceptable because the maternal characteristics of our study participants were similar to those in other studies conducted in other areas in Japan.41, 42 Second, although BP changes during pregnancy,43 the influence of longitudinal changes in BP on infant birth weight was not considered. A decrease in maternal BP from early- to mid-gestation is referred to as the ‘mid-pregnancy fall’.44 This phenomenon is thought to arise from the decrease of peripheral vascular resistance45 and tends to be associated with the clinical onset of pre-eclampsia.44 Thus, this phenomenon may affect fetal growth and infant’s birth weight. Further research about the association between mid-pregnancy fall and infant birth weight is needed. In addition, it was difficult to obtain comparisons of CBP and HBP in each trimester of pregnancy because only 193 women measured CBP and HBP during the first trimester. A larger sample size is needed to address this issue. Third, a comparison of the effect CBP vs. HBP on infant birth weight after 20 weeks’ gestation was not determined. BP evaluation after 20 weeks’ gestation is also important because PIH can develop in this period. Macdonald-Wallis et al.46 reported a negative association between the increase in maternal BP after 18 weeks’ gestation and infant birth weight in the Avon Longitudinal Study of Parents and Children (ALSPAC). We will examine this issue in the future. Fourth, other perinatal outcomes such as placental abruption, premature deliver and SGA infants could not be evaluated because the number of these events was too small. To clarify the impact of these outcomes, a larger sample size is needed. Fifth, diurnal variation of BP, which can be detected using 24-h ambulatory BP, was not assessed.47 The reason that we evaluated HBP but not ambulatory BP as an alternative to CBP in our study was described previously.26 Although health-care providers seem to prefer to use HBP over ambulatory BP in the field,48, 49 comparison of effects between ambulatory BP and other BP measurements on perinatal outcome may be needed. Sixth, in general, maternal BP changes from preconception to early pregnancy.50 Although it is important to evaluate the effects between HBP and CBP measured before conception on infant’s birth weight and Mahendru et al.51 recently reported that it was feasible to recruit women before conception, we were not able to collect the data before conception including BP because almost all women were recruited after diagnosis of intrauterine pregnancy. A prospective cohort study that recruits women before conception is needed to evaluate this issue. Seventh, we could not evaluate the effect of variability in HBP on infant birth weight because the numbers of HBP measurements were restricted to compare HBP and CBP in this study. Previous study reported variability in HBP was predictive of cardiovascular events in general population.52 Therefore, variability in HBP during pregnancy may also be associated with perinatal outcomes. The other approach is needed to examine the association between variability in HBP and perinatal outcomes.
In conclusion, high maternal home DBP and MAP before 20 weeks’ gestation was associated with a higher risk of lower infant birth weight than clinic DBP and MAP. HBP may be better than CBP to reflect increases in maternal peripheral vascular resistance. Therefore, in addition to CBP, it may be worth having pregnant women measure HBP to determine the risk of lower infant birth weight.
References
Gluckman PD, Hanson MA . Living with the past: evolution, development, and patterns of disease. Science 2004; 305: 1733–1736.
de Boo HA, Harding JE . The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol 2006; 46: 4–14.
Ay L, Kruithof CJ, Bakker R, Steegers EA, Witteman JC, Moll HA, Hofman A, Mackenbach JP, Hokken-Koelega AC, Jaddoe VW . Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. The Generation R Study. BJOG 2009; 116: 953–963.
Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN . A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol 2009; 201: 339.e331–314.
Bakker R, Kruithof C, Steegers EA, Tiemeier H, Mackenbach JP, Hofman A, Jaddoe VW . Assessment of maternal smoking status during pregnancy and the associations with neonatal outcomes. Nicotine Tob Res 2011; 13: 1250–1256.
Bakker R, Steegers EA, Hofman A, Jaddoe VW . Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: the generation R study. Am J Epidemiol 2011; 174: 797–806.
Beltran AJ, Wu J, Laurent O . Associations of meteorology with adverse pregnancy outcomes: a systematic review of preeclampsia, preterm birth and birth weight. Int J Environ Res Public Health 2014; 11: 91–172.
Hutcheon JA, Lisonkova S, Joseph KS . Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25: 391–403.
Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM . The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 2001; 20: Ix–xiv.
Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ . Maternal preeclampsia and neonatal outcomes. J Pregnancy 2011; 2011: 214365.
Ghulmiyyah L, Sibai B . Maternal mortality from preeclampsia/eclampsia. Semin Perinatol 2012; 36: 56–59.
Ostlund E, Al-Nashi M, Hamad RR, Larsson A, Eriksson M, Bremme K, Kahan T . Normalized endothelial function but sustained cardiovascular risk profile 11 years following a pregnancy complicated by preeclampsia. Hypertens Res 2013; 36: 1081–1087.
Poon LC, Kametas NA, Valencia C, Chelemen T, Nicolaides KH . Hypertensive disorders in pregnancy: screening by systolic diastolic and mean arterial pressure at 11-13 weeks. Hypertens Pregnancy 2011; 30: 93–107.
Hirashima C, Ohkuchi A, Takahashi K, Suzuki H, Matsuda Y, Matsubara S, Suzuki M . Additive effects of mean blood pressure and bilateral notching in the second trimester on subsequent angiogenesis-related factors. Hypertens Res 2014; 37: 76–81.
Niiranen TJ, Hanninen MR, Johansson J, Reunanen A, Jula AM . Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension 2010; 55: 1346–1351.
Fagard RH, Van Den Broeke C, De Cort P . Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens 2005; 19: 801–807.
Bobrie G, Chatellier G, Genes N, Clerson P, Vaur L, Vaisse B, Menard J, Mallion JM . Cardiovascular prognosis of "masked hypertension" detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA 2004; 291: 1342–1349.
Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G . Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005; 111: 1777–1783.
Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Kikuya M, Ito S, Satoh H, Hisamichi S . Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens 1998; 16: 971–975.
Lo C, Taylor RS, Gamble G, McCowan L, North RA . Use of automated home blood pressure monitoring in pregnancy: is it safe? Am J Obstet Gynecol 2002; 187: 1321–1328.
Ochsenbein-Kolble N, Roos M, Gasser T, Huch R, Huch A, Zimmermann R . Cross sectional study of automated blood pressure measurements throughout pregnancy. BJOG 2004; 111: 319–325.
Denolle T, Daniel JC, Calvez C, Ottavioli JN, Esnault V, Herpin D . Home blood pressure during normal pregnancy. Am J Hypertens 2005; 18: 1178–1180.
Metoki H, Ohkubo T, Watanabe Y, Nishimura M, Sato Y, Kawaguchi M, Hara A, Hirose T, Obara T, Asayama K, Kikuya M, Yagihashi K, Matsubara Y, Okamura K, Mori S, Suzuki M, Imai Y, Group BS. Seasonal trends of blood pressure during pregnancy in Japan: the babies and their parents' longitudinal observation in Suzuki Memorial Hospital in Intrauterine Period study. J Hypertens 2008; 26: 2406–2413.
Karagiannis G, Akolekar R, Sarquis R, Wright D, Nicolaides KH . Prediction of small-for-gestation neonates from biophysical and biochemical markers at 11-13 weeks. Fetal Diagn Ther 2011; 29: 148–154.
Metoki H, Ohkubo T, Obara T, Akutsu K, Yamamoto M, Ishikuro M, Sakurai K, Iwama N, Katagiri M, Sugawara J, Hirose T, Sato M, Kikuya M, Yagihashi K, Matsubara Y, Yaegashi N, Mori S, Suzuki M, Imai Y, Group BS. Daily serial hemodynamic data during pregnancy and seasonal variation: the BOSHI study. Clin Exp Hypertens 2012; 34: 290–296.
Ishikuro M, Obara T, Metoki H, Ohkubo T, Yamamoto M, Akutsu K, Sakurai K, Iwama N, Katagiri M, Yagihashi K, Yaegashi N, Mori S, Suzuki M, Kuriyama S, Imai Y . Blood pressure measured in the clinic and at home during pregnancy among nulliparous and multiparous women: the BOSHI study. Am J Hypertens 2013; 26: 141–148.
Brown MA, Roberts L, Davis G, Mangos G . Can we use the Omron T9P automated blood pressure monitor in pregnancy? Hypertens Pregnancy 2011; 30: 188–193.
McCullagh P . Regression models for ordinal data. J R Stat Soc Series B Stat Methodol 1980; 42: 109–142.
Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D . Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001; 103: 1245–1249.
Li Y, Wei FF, Thijs L, Boggia J, Asayama K, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Ohkubo T, Jeppesen J, Gu YM, Torp-Pedersen C, Dolan E, Liu YP, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Mena L, Maestre GE, Filipovsky J, Imai Y, O'Brien E, Wang JG, Staessen JA . Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation 2014; 130: 466–474.
Mook-Kanamori DO, Steegers EA, Eilers PH, Raat H, Hofman A, Jaddoe VW . Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA 2010; 303: 527–534.
McEniery CM, Yasmin, Wallace S, Maki-Petaja K, McDonnell B, Sharman JE, Retallick C, Franklin SS, Brown MJ, Lloyd RC, Cockcroft JR, Wilkinson IB, Investigators ES. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension 2005; 46: 221–226.
Pijnenborg R, Vercruysse L, Hanssens M . The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006; 27: 939–958.
Dugoff L, Lynch AM, Cioffi-Ragan D, Hobbins JC, Schultz LK, Malone FD, D'Alton ME . Consortium FTR. First trimester uterine artery Doppler abnormalities predict subsequent intrauterine growth restriction. Am J Obstet Gynecol 2005; 193: 1208–1212.
Melchiorre K, Leslie K, Prefumo F, Bhide A, Thilaganathan B . First-trimester uterine artery Doppler indices in the prediction of small-for-gestational age pregnancy and intrauterine growth restriction. Ultrasound Obstet Gynecol 2009; 33: 524–529.
Valensise H, Novelli GP, Vasapollo B, Borzi M, Arduini D, Galante A, Romanini C . Maternal cardiac systolic and diastolic function: relationship with uteroplacental resistances. A Doppler and echocardiographic longitudinal study. Ultrasound Obstet Gynecol 2000; 15: 487–497.
Vasapollo B, Novelli GP, Valensise H . Total vascular resistance and left ventricular morphology as screening tools for complications in pregnancy. Hypertension 2008; 51: 1020–1026.
Safar ME, Boudier HS . Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension 2005; 46: 205–209.
Wei JN, Sung FC, Li CY, Chang CH, Lin RS, Lin CC, Chiang CC, Chuang LM . Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in Taiwan. Diabetes Care 2003; 26: 343–348.
Boulet SL, Alexander GR, Salihu HM, Pass M . Macrosomic births in the United States: determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol 2003; 188: 1372–1378.
Iwasaki R, Ohkuchi A, Furuta I, Ojima T, Matsubara S, Sato I, Minakami H . Relationship between blood pressure level in early pregnancy and subsequent changes in blood pressure during pregnancy. Acta Obstet Gynecol Scand 2002; 81: 918–925.
Takimoto H, Sugiyama T, Nozue M, Kusama K, Fukuoka H, Kato N, Yoshiike N . Maternal antenatal body mass index gains as predictors of large-for-gestational-age infants and cesarean deliveries in Japanese singleton pregnancies. J Obstet Gynaecol Res 2011; 37: 553–562.
Fujime M, Tomimatsu T, Okaue Y, Koyama S, Kanagawa T, Taniguchi T, Kimura T . Central aortic blood pressure and augmentation index during normal pregnancy. Hypertens Res 2012; 35: 633–638.
Silva LM, Steegers EA, Burdorf A, Jaddoe VW, Arends LR, Hofman A, Mackenbach JP, Raat H . No midpregnancy fall in diastolic blood pressure in women with a low educational level: the Generation R Study. Hypertension 2008; 52: 645–651.
Duvekot JJ, Cheriex EC, Pieters FA, Menheere PP, Peeters LH . Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol 1993; 169: 1382–1392.
Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA . Associations of blood pressure change in pregnancy with fetal growth and gestational age at delivery: findings from a prospective cohort. Hypertension 2014; 64: 36–44.
Hermida RC, Ayala DE, Mojon A, Fernandez JR, Alonso I, Silva I, Ucieda R, Iglesias M . Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension 2000; 36: 149–158.
Dehaeck U, Thurston J, Gibson P, Stephanson K, Ross S . Blood pressure measurement for hypertension in pregnancy. J Obstet Gynaecol Can 2010; 32: 328–334.
Imai Y, Obara T, Asamaya K, Ohkubo T . The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res 2013; 36: 661–672.
Mahendru AA, Everett TR, Wilkinson IB, Lees CC, McEniery CM . Maternal cardiovascular changes from pre-pregnancy to very early pregnancy. J Hypertens 2012; 30: 2168–2172.
Mahendru AA, Everett TR, McEniery CM, Wilkinson IB, Lees CC . The feasibility of prospectively studying maternal cardiovascular changes from before conception. Hypertens Res 2013; 36: 698–704.
Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y . Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension 2008; 52: 1045–1050.
Acknowledgements
This study was supported by Grants for Scientific Research (18590587, 18390192, 21390201, 25253059 and 26860412) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; a Grant-in-Aid (H21-Junkankitou [Seishuu]-Ippan-004) from the Ministry of Health, Labor and Welfare, Health and Labor Sciences Research Grants, Japan; a Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) fellows (19.7152, 20.7198, 20.7477 and 20.54043); grants from the Takeda Science Foundation; and grants from the OTC Self-Medication Promotion Foundation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
HM and YI have taken part in, and are currently involved with, collaborative research with Omron Healthcare.
Additional information
The BOSHI Study Group Medical members (Obstetrics): Noriyuki Iwama, Hidekazu Nishigori, Kohei Tanaka, Takashi Sugiyama, Junichi Sugawara, Nobuo Yaegashi, Kazuhiko Hoshi, Kunihiko Okamura, Masakuni Suzuki. Medical members (Internal Medicine): Hirohito Metoki, Kei Asayama, Ryusuke Inoue, Masahiro Kikuya, Takayoshi Ohkubo, Shinichi Kuriyama, Yutaka Imai. Coordinating members: Taku Obara, Mami Ishikuro, Rie Tsuchida, Azusa Hara, Takuo Hirose, Takeshi Kobayashi, Kenta Gonokami, Takanao Hashimoto, Yumiko Watanabe, Misato Nishimura, Maiko Kawaguchi, Yurie Sato, Minako Hoshikawa, Ayano Sasaki, Kasumi Sakurai, Michihiro Sato, Konomi Akutsu, Mami Yamamoto, Aya shiraishi, Miki Hosaka. Clinical examination members: Ikuo Tachibana, Maki Omura, Mikiko Ishikawa, Yoshimi Fujii, Hidemi Kobayashi, Kazuyuki Akaishi. Pharmaceutical members: Yuko Kikuchi, Kei Tate, Chieko Koishi, Saori Sugawara. Recruitment members: Katsuyo Yagihashi, Junko Saitou, Hiromi Sasaki, Tomoko Suzuki, Junko Takahashi, Yoko Narita, Satoko Shigihara, Hideko Tada, Yumi Hamada. Outpatient management members: Nozomi Satou. Nami Satou, Setsuko Sai, Nana Atsumi, Naoko Sekine, Yukari Ueno, Yu Itou. Inpatient management members: Yukie Obara, Nami Onodera, Asako Sato, Youko Iwasa, Mamiko Abe, Yukari Kido, Risako Komuro, Yukiko Nakamura, Marie Watanabe, Chikako Matsumoto, Koto Oyama, Aya Takahashi, Michiko Kojima, Miyuki Abe, Mariko Sane, Mana Takahashi, Kana Sugata Miho Igari, Haruhi Sasaki, Mizuki Kobayashi, Aya Kikuchi, Risa Yamamoto, Akiho Goto, Eri Yamauchi, Mika Chiba, Sakiko Ota, Hiromi Ishikawa, Akemi Sasaki, Tomoko Kawamura, Hiroko Hiji, Misaki Kishinami, Yurie Kowata, Eiko Yamauchi, Yasuko Takahashi, Naho Sato.
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Iwama, N., Metoki, H., Ohkubo, T. et al. Maternal clinic and home blood pressure measurements during pregnancy and infant birth weight: the BOSHI study. Hypertens Res 39, 151–157 (2016). https://doi.org/10.1038/hr.2015.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.108
Keywords
This article is cited by
-
Self-monitoring of blood pressure among women with hypertensive disorders of pregnancy: a systematic review
BMC Pregnancy and Childbirth (2022)
-
Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement
Hypertension Research (2022)
-
A personal history of research on hypertension From an encounter with hypertension to the development of hypertension practice based on out-of-clinic blood pressure measurements
Hypertension Research (2022)
-
Risk scores for predicting small for gestational age infants in Japan: The TMM birthree cohort study
Scientific Reports (2022)
-
Association of maternal home blood pressure trajectory during pregnancy with infant birth weight: the BOSHI study
Hypertension Research (2020)