Abstract
Renal superoxide excess, which is induced by an imbalance of the superoxide-producing enzyme NAD(P)H oxidase and the superoxide-scavenging enzyme superoxide dismutase (SOD) under hyperglycemia, increases oxidative stress and contributes to the development of diabetic nephropathy. In this study, we treated non-obese and hypoinsulinemic C57BL/6-Ins2Akita (C57BL/6-Akita) diabetic mice with telmisartan (5 mg kg−1 per day), an angiotensin II type 1 receptor blocker, or amlodipine (5 mg kg−1 per day), a calcium channel blocker, for 4 weeks and compared the effects of these two anti-hypertensive drugs on renal NAD(P)H oxidase, SOD and transcription factor Nrf2 (NF-E2-related factor 2), which is known to upregulate several antioxidant enzymes including SOD. Vehicle-treated C57BL/6-Akita mice exhibited higher renal NAD(P)H oxidase and lower renal SOD activity with increased levels of renal superoxide than the C57BL/6-wild-type non-diabetic mice. Interestingly, telmisartan treatment not only reduced NAD(P)H oxidase activity but also enhanced SOD activity in C57BL/6-Akita mouse kidneys, leading to a reduction of renal superoxide levels. Furthermore, telmisartan-treated C57BL/6-Akita mice increased the renal protein expression of SOD and Nrf2. In parallel with the reduction of renal superoxide levels, a reduction of urinary albumin levels and a normalization of elevated glomerular filtration rate were observed in telmisartan-treated C57BL/6-Akita mice. In contrast, treatment with amlodipine failed to modulate renal NAD(P)H oxidase, SOD and Nrf2. Finally, treatment of C57BL/6-Akita mice with apocynin, an NAD(P)H oxidase inhibitor, also increased the renal protein expression of SOD and Nrf2. Collectively, our data suggest that NAD(P)H oxidase negatively regulates renal SOD, possibly by downregulation of Nrf2, and that telmisartan could upregulate renal SOD by the suppression of NAD(P)H oxidase and subsequent upregulation of Nrf2, leading to the amelioration of renal oxidative stress and diabetic renal changes.
Similar content being viewed by others
Introduction
Growing evidence indicates that superoxide anion (O2•−) excess induced by chronic hyperglycemia contributes to glomerular injury, which characterizes diabetic nephropathy (DN) through the formation of secondary reactive oxygen species including peroxynitrite and hydroxyl radicals.1, 2, 3, 4 Two endogenous enzymes, NAD(P)H oxidase and superoxide dismutase (SOD), are thought to be key determinants for the superoxide anion levels in kidneys. NAD(P)H oxidase is the most important source of superoxide anions,5, 6, 7, 8 whereas SOD is a major defender against superoxide anions.9, 10 SOD consists of three enzymatic isoforms: cytosolic CuZn-SOD (SOD1), mitochondrial Mn-SOD (SOD2), and extracellular CuZn-SOD (SOD3).10, 11 These three isoforms are derived from distinct genes but catalyze the same reaction.11 Each SOD isoform neutralizes superoxide anions into hydrogen peroxide (H2O2) and molecular oxygen,9, 10 followed by the reduction of hydrogen peroxide to water (H2O) by catalase in peroxisomes or glutathione peroxidase in mitochondria.1, 12 Thus, SOD serves as a major antioxidant enzyme responsible for the first step of the superoxide removal system. Recent studies of streptozotocin-induced diabetic rats have shown that chronic hyperglycemia enhance NAD(P)H oxidase activity in kidneys.4, 13, 14 Furthermore, we have more recently demonstrated that chronic hyperglycemia causes a reduction of renal SOD expression and activity in a mouse model susceptible to the development of DN.15 On the basis of these findings, renal alterations of NAD(P)H oxidase and SOD enzymes are likely to be responsible for the renal superoxide excess observed in chronic hyperglycemia.
Angiotensin II is an important vasoconstrictor that regulates systemic and glomerular hemodynamics. In addition, angiotensin II is also known to promote sodium reabsorption, cell growth and extracellular matrix deposition in kidneys.16 These effects are generally triggered by signaling via angiotensin II type 1 (AT1) receptors.16 Therefore, AT1 receptor blockade not only improves systemic hypertension but also could provide direct renoprotective effects. Indeed, AT1 receptor blockers (ARBs) have been shown to exert greater renoprotective effects in patients with DN than other classes of anti-hypertensive drugs. Clinical studies have reported that irbesartan, an ARB, significantly inhibits the aggravation of renal function in type 2 diabetic patients with overt DN compared with amlodipine,17 a calcium channel blocker, and that valsartan, another ARB, significantly reduces albuminuria in type 2 diabetic patients with incipient DN compared with amlodipine.18 It is noteworthy that the renoprotective effects of ARBs were independent of their systemic blood pressure-lowering properties. Recently, evidence for the anti-oxidative effects of ARBs has been accumulating for several members of the ARB class. Clinical studies have reported that ARBs, including losartan, candesartan, olmesartan, telmisartan and valsartan, reduce the urinary levels of oxidative stress marker 8-hydroxy-2′-deoxyguanosine in patients with DN.19, 20 Oxidative stress induced by superoxide excess under chronic hyperglycemia has been thought to be a major factor involved in the pathogenesis of DN.21 Therefore, the powerful renoprotective properties of ARBs may be attributed to their antioxidative effects.
The role of angiotensin II in modulating superoxide-producing enzyme NAD(P)H oxidase has been explored in vascular cells and kidneys. Previous experimental studies have demonstrated that angiotensin II promotes superoxide generation via NAD(P)H oxidase activation in vascular cells.22, 23 Furthermore, recent experimental studies have indicated that ARBs, such as olmesartan and telmisartan, reduce glomerular superoxide production through downregulating the gene expression of NAD(P)H oxidase subunits p22phox and p47phox in subtotal nephrectomized rats24 and catalase-deficient acatalasemic mice.25 However, whether the angiotensin II signaling and its inhibition modulate the superoxide-scavenging SOD enzymes in kidneys exposed to hyperglycemia is largely unknown.
To investigate whether AT1 receptor blockade alters the expression and activity of SOD enzymes in kidneys exposed to hyperglycemia, we treated non-obese and hypoinsulinemic C57BL/6-Ins2Akita (C57BL/6-Akita) diabetic mice with telmisartan, an ARB, or amlodipine, a calcium channel blocker, and compared the effects of these two anti-hypertensive drugs on renal SOD. We report here that telmisartan not only reduces renal NAD(P)H oxidase activity but also upregulates the renal expression and activity of SOD, resulting in a reduction of renal superoxide levels. To explore the mechanism underlying this upregulation of renal SOD with telmisartan, we further examined whether NAD(P)H oxidase inhibition with apocynin modulated the renal expression of SOD and redox-sensitive transcription factor Nrf2 (NF-E2-related factor 2), which is known to upregulate several antioxidant enzymes, including SOD,26, 27, 28 in C57BL/6-Akita diabetic mice.
Methods
Experimental animals
Male C57BL/6-Akita diabetic and C57BL/6-wild-type (C57BL/6-WT) non-diabetic mice were purchased from SLC (Shizuoka, Japan). The mice were housed (n=3–4 per cage) in a room with a relative humidity of 50% and a 12/12-h light/dark cycle at 20–22 °C, and had unrestricted access to standard rodent chow and water. Animal experiments were conducted in accordance with the Animal Welfare Guidelines of Akita University. All procedures were approved by the Committee on Animal Experimentation of Akita University.
Treatment protocols for telmisartan, amlodipine and apocynin
Telmisartan was kindly provided by Boehringer Ingelheim (Tokyo, Japan). Amlodipine, apocynin (4′-hydroxy-3′-methoxyacetophenone) and carboxymethylcellulose-Na were purchased from Sigma-Aldrich (St Louis, MO, USA). To investigate the effects of telmisartan and amlodipine on renal SOD and NAD(P)H oxidase, 10-week-old male C57BL/6-Akita diabetic mice were administered with telmisartan (5 mg kg−1) orally or amlodipine (5 mg kg−1) dissolved in a 0.5% carboxymethylcellulose-Na solution once daily for 4 weeks. Mice in the control group were given the same volume of 0.5% carboxymethylcellulose-Na solution alone as the vehicle. To examine the effects of NAD(P)H oxidase inhibition on renal SOD, 10-week-old male C57BL/6-Akita diabetic mice were treated with apocynin (40 mg kg−1 per day) for 8 weeks. Apocynin was added in the drinking water and was administered orally to the mice as described previously.29 Mice in the control group were given water alone as the vehicle.
Measurement of blood and urine parameters
Blood glucose was measured using Glucocard Diameter (Arkray, Tokyo, Japan) on samples obtained after a 6-h daytime fast. Blood urea nitrogen, total plasma cholesterol and plasma triglycerides were enzymatically measured by an autoanalyzer (Fuji Dry-Chem 5500, Fuji Film, Tokyo, Japan). Urinary albumin excretion was assessed by determining the albumin-to-creatinine ratio in morning spot urine as previously described.30 Urine albumin and creatinine were measured by an Albuwell-M Murine Microalbuminuria ELISA kit and a Creatinine Companion kit, respectively (Exocell, Philadelphia, PA, USA).
Measurement of physiological parameters and renal histologic analysis
Systolic blood pressure was measured in conscious trained mice at room temperature using a non-invasive tail cuff and a pulse transducer system (BP-98A, Softron, Tokyo, Japan). The glomerular filtration rate (GFR) was measured by a single-bolus fluorescein isothiocyanate-inulin injection method as described previously.31 For renal histologic analysis, we stained kidney sections with periodic acid-Schiff (PAS). We used a semi-quantitative score to evaluate the degree and extent of glomerular mesangial expansion as described previously.30
Renal superoxide production and activity of NAD(P)H oxidase and SOD
Renal superoxide levels were assessed by dihydroethidium (DHE) histochemistry and a water-soluble tetrazolium salt (WST-1, 2-[4-iodophenyl]-3-[4-nitrophenyl]-5-[2,4-disulfophenyl]-2H-tetrazolium) reduction assay as previously described.15 The specificity of the WST-1 assay was confirmed by pretreating 10 mg of kidney tissue with polyethylene glycol-SOD (20U; Sigma-Aldrich) overnight at 37 °C. Renal superoxide levels were expressed as the absorbance at 450 nm per 10 mg tissue. For the DHE staining, we examined the Eth-DNA fluorescence at 480 nm excitation and 610 nm emission. To measure renal NAD(P)H oxidase and SOD activity, we prepared kidney lysates using phosphate-buffered saline-perfused and freshly removed renal cortical tissue as previously described.15 Renal NAD(P)H oxidase activity was measured by a lucigenin-enhanced chemiluminescence assay as previously described.14 Renal SOD activity was determined using an SOD assay kit-WST (Dojindo Molecular Technologies, Gaithersburg, MD, USA) as previously described.15 The protein amount was measured using a bicinochoninic acid protein assay (Sigma-Aldrich). The enzymatic activity of NAD(P)H and SOD were expressed in relative chemiluminescence (light) units (RLU) per 100 μg protein and units per mg protein, respectively.
Western blot analysis and immunohistochemistry
For western blot analysis, the kidney lysate prepared for the SOD activity measurement was used. In all, 20 μg of protein was separated by SDS-PAGE and subjected to immunoblots. As the primary antibody, we used rabbit anti-CuZn-SOD (SOD1) (1 : 10 000; Stressgen, Ann Arbor, MI, USA), anti-Mn-SOD (SOD2) (1 : 10 000; Stressgen), anti-EC-SOD (SOD3) (1 : 2000; Stressgen) or anti-Nrf2 (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) polyclonal antibodies. The loading of lysate protein was evaluated by an immunoblot using rabbit anti-actin antibody (1 : 1000; Sigma-Aldrich). The intensity of the signals was semi-quantified using Adobe Photoshop (version CS4; Adobe Systems, San Jose, CA, USA). For the immunohistochemistry, cryostat sections were prepared as previously described.15 We labeled the sections with rabbit anti-CuZn-SOD (SOD1) (1 : 100; Stressgen), anti-Mn-SOD (SOD2) (1 : 100; Stressgen) or anti-EC-SOD (SOD3) (1 : 50; Stressgen) polyclonal antibodies for 1 h at room temperature, followed by Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (1 : 200; Molecular Probes, Eugene, OR, USA) for 30 min at room temperature. We then counterstained the sections with ToPro-3 (Molecular Probes).
Measurement of renal prostaglandin E2 (PGE2) levels
Renal PGE2 levels were measured using freshly isolated renal cortical tissue as previously described.32 The levels were expressed as the ratio of renal cortical PGE2 to protein.
Statistical analysis
Data were presented as the mean±s.e.m. Statistical analyses were conducted using GraphPad Prism software (GraphPad, San Diego, CA, USA). Differences between groups were determined by an unpaired t-test or a one-way ANOVA, followed by Bonferroni’s multiple comparison test. A P<0.05 was considered statistically significant.
Results
Biochemical and physiological parameters, and renal histopathology after a 4-week treatment with telmisartan or amlodipine in C57BL/6-Akita diabetic mice
Table 1 shows the biochemical and physiological parameters after a 4-week treatment with telmisartan or amlodipine in C57BL/6-Akita diabetic mice. Data from telmisartan-treated mice were compared with those from the mice treated with amlodipine. Treatment with either telmisartan or amlodipine did not affect blood glucose, body weight, blood urea nitrogen, total cholesterol or triglyceride levels. Compared with the vehicle-treated mice, both amlodipine-treated and telmisartan-treated mice exhibited significantly lower blood pressure levels. There were no significant differences in blood pressure levels between amlodipine-treated and telmisartan-treated mice. Compared with the data from vehicle-treated mice, treatment with telmisartan significantly reduced urinary albumin levels, GFR, and the left kidney weight-to-body weight ratio (LKW/BW) in C57BL/6-Akita diabetic mice, whereas treatment with amlodipine had no effect. As we recently reported, glomerular pathological damages were relatively mild in 10–15-week-old C57BL/6-Akita diabetic mice.15 A difference in glomerular pathological changes evaluated using the mesangial expansion score was not observed between vehicle-treated and telmisartan-treated C57BL/6-Akita diabetic mice.
Effects of treatment with telmisartan on renal superoxide production, NAD(P)H oxidase activity and SOD activity in C57BL/6-Akita diabetic mice
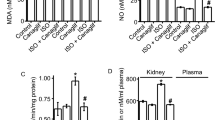
Figure 1 shows renal superoxide production, NAD(P)H oxidase activity and SOD activity after a 4-week treatment with telmisartan or amlodipine in C57BL/6-Akita diabetic mice. Renal superoxide levels were assessed by DHE histochemistry and a WST-1 assay. The DHE fluorescence signal, which reflects superoxide production, was decreased in the glomeruli of telmisartan-treated C57BL/6-Akita diabetic mice compared with vehicle-treated mice (Figure 1A). Furthermore, telmisartan but not amlodipine significantly reduced renal superoxide levels as determined by the WST-1 assay in C57BL/6-Akita diabetic mice (Figure 1B). Consistent with a recent report,33 telmisartan reduced NAD(P)H activity in the kidneys of C57BL/6-Akita diabetic mice close to the levels of C57BL/6-wild-type (C57BL/6-WT) non-diabetic mice (Figure 1C). Interestingly, telmisartan-treated C57BL/6-Akita diabetic mice but not amlodipine-treated mice showed significantly higher renal SOD activity than the vehicle-treated mice (Figure 1D). Thus, it should be noted that two telmisartan-treated and amlodipine-treated C57BL/6-Akita diabetic mouse groups exhibited different levels of renal NAD(P)H and SOD activity, despite comparable levels of hyperglycemia and blood pressure.
Effects of telmisartan treatment on renal superoxide production, NAD(P)H oxidase activity and SOD activity in C57BL/6-Akita diabetic mice. The C57BL/6-Akita diabetic mice were treated with the vehicle, amlodipine or telmisartan for 4 weeks. The treatment started at 10 weeks of age and ended at 14 weeks of age. The data from C57BL/6-Akita diabetic mice were compared with those from age-matched C57BL/6-wild-type non-diabetic mice. (A) Representative glomerular DHE staining after a 4-week treatment with telmisartan. (a) C57BL/6-wild-type; (b) vehicle-treated C57BL/6-Akita; (c) amlodipine-treated C57BL/6-Akita; (d) telmisartan-treated C57BL/6-Akita. (B) Renal superoxide production after a 4-week treatment with telmisartan. Data are presented as the mean±s.e.m. SOD+, kidney tissue pre-incubated with SOD-PEG protein; SOD−, kidney tissue without SOD-PEG protein. n=5 per group. *P<0.001 vs. wild-type. +P<0.01 vs. vehicle. (C) Renal NAD(P)H oxidase activity after a 4-week treatment with telmisartan. Data are presented as the mean±s.e.m. n=5 per group. *P<0.001 vs. wild-type. ‡P<0.001 vs. vehicle. (D) Renal SOD activity after a 4-week treatment with telmisartan. Data are presented as the mean±s.e.m. n=5 per group. *P<0.001 vs. wild-type. ‡P<0.001 vs. vehicle.
Effects of treatment with telmisartan on the renal expression of SOD isoforms and Nrf2 in C57BL/6-Akita diabetic mice
We next examined whether a 4-week treatment with telmisartan affected the renal expression of SOD isoforms and Nrf2. Western blot analysis revealed increased expression of the SOD isoforms SOD1, SOD2, and SOD3 and Nrf2 in the renal cortex of telmisartan-treated C57BL/6-Akita diabetic mice, whereas renal SOD and Nrf2 upregulation was not observed in amlodipine-treated mice (Figure 2). Figure 3 shows the renal SOD isoform expression using immunofluorescence histochemistry. As shown in our recent report,15 SOD1 and SOD2 were broadly expressed in glomerular and tubular cells (tubular SOD1 expression not shown). SOD3 expression was observed in glomerular capillaries and the arteriolar wall (expression of SOD3 in the arteriolar wall not shown). Consistent with the results of the western blot analysis, strong SOD1 and SOD3 signals in the glomeruli and SOD2 signals in proximal tubules were observed in telmisartan-treated C57BL/6-Akita diabetic mice. In contrast, the kidneys of amlodipine-treated C57BL/6-Akita diabetic mice did not exhibit increased expression of SOD isoforms.
Western blot analysis of renal cortical SOD isoforms and Nrf2 expression after a 4-week treatment with the vehicle (VE), amlodipine (AM) or telmisartan (TE) in C57BL/6-Akita diabetic mice. WT indicates non-diabetic C57BL/6-wild-type mice. The relative intensity of the SOD-to-actin or Nrf2-to-actin ratios to WT is also shown in the lower panels. Data are presented as the mean±s.e.m. n=4 per group. *P<0.001 vs. VE.
Immunofluorescence SOD isoform staining of kidney sections after a 4-week treatment with the vehicle, amlodipine or telmisartan in C57BL/6-Akita diabetic mice. (a–d) SOD1; (e–h) SOD2; (i–l) SOD3; (a, e, i) C57BL/6-wild-type; (b, f, j) vehicle-treated C57BL/6-Akita; (c, g, k) amlodipine-treated C57BL/6-Akita; (d, h, l) telmisartan-treated C57BL/6-Akita.
NAD(P)H oxidase inhibition by treatment with apocynin and renal alterations of the SOD enzyme and Nrf2 in C57BL/6-Akita diabetic mice
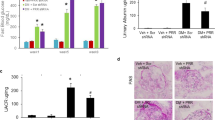
We found that telmisartan reduced renal superoxide levels through downregulating NAD(P)H oxidase and upregulating SOD in a mouse model of diabetes, C57BL/6-Akita mice. To explore whether NAD(P)H oxidase regulates the SOD enzyme via Nrf2 in kidneys, we treated C57BL/6-Akita diabetic mice with apocynin, an NAD(P)H oxidase inhibitor, for 8 weeks and investigated the renal alterations of the SOD enzyme and Nrf2. As shown in Table 2, apocynin treatment did not affect body weight, blood pressure, blood glucose or LKW/BW. As expected, a marked reduction of renal superoxide and NAD(P)H oxidase activity was observed in the apocynin-treated C57BL/6-Akita diabetic mice. In addition, apocynin significantly lowered urinary albumin levels and ameliorated elevated GFR in C57BL/6-Akita diabetic mice. Figure 4 shows the western blot analysis of renal cortical expression of SOD isoforms and Nrf2 after an 8-week treatment with apocynin. Interestingly, renal cortical expression levels of SOD1, SOD2 and SOD3 isoforms and Nrf2 were significantly increased in apocynin-treated C57BL/6-Akita diabetic mice compared with vehicle-treated mice.
Western blot analysis of renal cortical SOD isoform and Nrf2 expression after an 8-week treatment with the vehicle (VE) or apocynin (AP) in C57BL/6-Akita diabetic mice. WT indicates non-diabetic C57BL/6-wild-type mice. The relative intensity of the SOD-to-actin or Nrf2-to-actin ratios to WT is also shown in the lower panels. Data are presented as the mean±s.e.m. n=4 per group. *P<0.001 vs. VE.
Effects of telmisartan and apocynin on renal production of vasodilatory PGE2 in C57BL/6-Akita diabetic mice
To elucidate the mechanism through which superoxide reduction due to treatment with telmisartan or apocynin affects glomerular hemodynamics, we measured renal cortical levels of vasodilatory PGE2, which contributes to the development of glomerular hypertension in C57BL/6-Akita diabetic mouse groups treated with telmisartan for 4 weeks and with apocynin for 8 weeks. As shown in Figure 5, a reduction of renal PGE2 production was observed in the telmisartan-treated and apocynin-treated groups compared with the vehicle-treated group.
Discussion
Telmisartan is a unique ARB with peroxisome proliferator-activated receptor-γ activity,34 and this ARB has been reported to offer a more powerful antioxidative effect than other members of the ARB class in patients with DN.20 In this study, we first treated 10-week-old C57BL/6-Akita diabetic mice with telmisartan for 4 weeks and investigated whether AT1 receptor blockade by telmisartan modulated the superoxide-scavenging SOD enzyme in kidneys exposed to hyperglycemia. C57BL/6-Akita mice are well-characterized as a model of non-obese and hypoinsulinemic diabetes, and develop marked hyperglycemia as early as 4 weeks of age because of a single mutation in cysteine 96 to tyrosin in the insulin 2 gene (Ins2Akita).35, 36 Chemicals such as streptozotocin or alloxan are widely used to induce diabetes in experimental animals. However, these chemicals have been documented to generate reactive oxygen species and enhance oxidative stress.37 Therefore, the C57BL/6-Akita mouse model offers a unique opportunity to precisely assess the renal alterations of oxidative stress and its related enzymes.
Consistent with the results of clinical studies,17, 18 our data also indicate that an ARB, telmisartan, is superior to a calcium channel blocker, amlodipine, at producing renoprotective effects in C57BL/6-Akita diabetic mice. In the SMART study, it was reported that changes in the urinary albumin-to-creatinine ratio from baseline to the end of the treatment period were −36% in type 2 diabetic and microalbuminuric patients treated with valsartan, an ARB, for 24 weeks and +30% in those treated with amlodipine for 24 weeks, despite comparable blood pressure reductions.38 These results indicate that monotherapy with amlodipine may be insufficient for albumin-to-creatinine ratio reduction. However, the SMART study also suggested that if blood pressure is sufficiently lowered by amlodipine treatment, albumin-to-creatinine ratio would also be reduced to some extent. Similar to the results of the SMART study, this study showed that treatment with telmisartan, not amlodipine, reduced urinary albumin levels and normalized elevated GFR that reflects glomerular hypertension in C57BL/6-Akita diabetic mice, despite comparable levels of hyperglycemia and blood pressure between the two groups treated with these drugs. Further blood pressure reduction with amlodipine treatment may be needed to reduce urinary albumin and glomerular hypertension in C57BL/6-Akita diabetic mice.
In this study, we used the tail cuff method to measure blood pressure. Our data did not indicate a difference in blood pressure between the telmisartan-treated and amlodipine-treated C57BL/6-Akita diabetic mice. However, the tail cuff method has several limitations in blood pressure measurement. Therefore, measuring blood pressure with the telemetry method may be necessary to precisely assess the effects of anti-hypertensive drugs on reducing blood pressure in C57BL/6-Akita diabetic mice.
Our experiments revealed the novel finding that telmisartan not only reduced NAD(P)H oxidase activity but also enhanced SOD activity in the kidneys of C57BL/6-Akita diabetic mice. As expected, renal alterations of these enzymes resulted in a reduction of renal superoxide levels. In contrast, treatment with amlodipine failed to modulate renal NAD(P)H oxidase and SOD enzymes. The differences in renoprotection between telmisartan and amlodipine may in part be attributed to their ability to modulate renal NAD(P)H oxidase and SOD enzymes. A recent experimental study reported that treatment with low doses of telmisartan (0.1–0.3 mg kg−1 per day) did not affect renal SOD activity in non-diabetic mice.25 However, our study indicated that treatment with a high dose of telmisartan (5 mg kg−1 per day) enhanced renal SOD activity in C57BL/6-Akita diabetic mice. It is possible that high doses of this ARB are needed to enhance renal SOD activity. Paralleling the elevation of renal SOD activity in telmisartan-treated C57BL/6-Akita diabetic mice, our immunohistochemical study revealed increases in the protein expression of SOD1 and SOD3 isoforms in glomeruli and of the SOD2 isoform in the proximal tubules of these mice. Western blot analysis confirmed the finding that telmisartan therapy increases the protein expression of SOD1, SOD2 and SOD3 in the kidneys of C57BL/6-Akita diabetic mice.
As angiotensin II signaling directly promotes NAD(P)H oxidase activation,22, 23 the downregulation of renal NAD(P)H oxidase by AT1 receptor blockade is a reasonable result. However, the mechanism by which telmisartan upregulates renal SOD remains unclear. We hypothesized that NAD(P)H oxidase would negatively regulate the renal expression of SOD or transcription factor Nrf2, which is known to upregulate several antioxidant enzymes including SOD.26, 27, 28 To test this hypothesis, we treated C57BL/6-Akita diabetic mice with an NAD(P)H oxidase inhibitor, apocynin, for 8 weeks and investigated the alteration of renal SOD and Nrf2 expression. As expected, apocynin treatment markedly lowered renal NAD(P)H oxidase activity in C57BL/6-Akita diabetic mice, resulting in the reduction of renal superoxide levels. The reduction of renal superoxide by apocynin therapy contributed to reducing urinary albumin levels and normalizing the elevated GFR in C57BL/6-Akita diabetic mice. Consistent with the results of recent experimental studies,29, 39 apocynin did not lower the blood pressure of C57BL/6-Akita diabetic mice. Although the cause is unclear, one possible explanation is that unlike telmisartan, apocynin is not effective in blocking systemic vasoconstriction by angiotensin II. More importantly, we found that inhibiting NAD(P)H oxidase upregulated renal SOD and Nrf2 in C57BL/6-Akita diabetic mice. Suppression of NAD(P)H oxidase by telmisartan also resulted in increased expression of renal Nrf2. Taken together, our data suggest that NAD(P)H oxidase negatively regulates renal SOD, possibly by downregulation of Nrf2, and that telmisartan could upregulate renal SOD by the suppression of NAD(P)H oxidase and subsequent upregulation of Nrf2 (Figure 6).
This study does not demonstrate that other members of the ARB class also have the ability to upregulate renal SOD. As AT1 receptor blockade by other members of the ARB class, such as olmesartan, has been shown to reduce renal NAD(P)H oxidase expression,24 all ARBs may share the renal SOD upregulating effect to some extent. However, as shown in a recent clinical study,20 there is a difference in the ability to ameliorate oxidative stress among the ARB members. Of the currently available ARBs, telmisartan is known to have the strongest binding affinity to AT1 receptors, the longest half-life, and a high lipophilicity.40, 41 In addition, telmisartan also acts as a partial agonist of peroxisome proliferator-activated receptor-γ. An experimental study of obese and hypertensive rats has indicated that peroxisome proliferator-activated receptor-γ activation by pioglitazone therapy downregulates NAD(P)H oxidase.42 These properties may explain the more powerful antioxidative and renoprotective effects of telmisartan by modulation of renal NAD(P)H oxidase and SOD.
Reactive oxygen species, including superoxide anions, induce overproduction of vasodilatory PGE2 through cyclooxygenase-2 upregulation.43 PGE2 is a vasodilator for afferent arterioles, and excessive PGE2 could cause the glomerular hypertension observed in early diabetes.44, 45 Therefore, it is thought that the reduction of renal superoxide with apocynin normalized elevated GFR in C57BL/6-Akita diabetic mice. In addition, renal superoxide reduction with telmisartan is likely to affect glomerular pressure reduction. This study revealed that treatment with apocynin or telmisartan reduces renal PGE2 production in C57BL/6-Akita diabetic mice. Furthermore, the ability of telmisartan to dilate efferent arterioles through AT1 receptor blockade also contributes to the amelioration of glomerular hypertension. Thus, renal superoxide reduction is thought to provide beneficial effects in improving abnormalities in glomerular hemodynamics in early diabetes.
In conclusion, we report a novel finding that AT1 receptor blockade by telmisartan treatment could upregulate renal SOD enzyme by the suppression of NAD(P)H oxidase and subsequent upregulation of Nrf2, leading to an improvement of oxidative stress in kidneys exposed to hyperglycemia. These effects are expected to greatly contribute to the amelioration of earlier diabetic renal change observed in C57BL/6-Akita diabetic mice.
References
Evans JL, Goldfine ID, Maddux BA, Grodsky GM . Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 2002; 23: 599–622.
Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, Forbes JM . RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol 2009; 20: 742–752.
Ghosh S, Khazaei M, Moien-Afshari F, Ang LS, Granville DJ, Verchere CB, Dunn SR, McCue P, Mizisin A, Sharma K, Laher I . Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am J Physiol Renal Physiol 2009; 296: F700–F708.
Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM . Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes 2008; 57: 460–469.
Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM . Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 2002; 105: 1656–1662.
Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, Sasaki T, Tsujioka K, Makino H, Kashihara N . NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol 2005; 288: F1144–F1152.
Soccio M, Toniato E, Evangelista V, Carluccio M, De Caterina R . Oxidative stress and cardiovascular risk: the role of vascular NAD(P)H oxidase and its genetic variants. Eur J Clin Invest 2005; 35: 305–314.
Wardle EN . Cellular oxidative processes in relation to renal disease. Am J Nephrol 2005; 25: 13–22.
Fridovich I . Superoxide radical and superoxide dismutases. Annu Rev Biochem 1995; 64: 97–112.
Fridovich I . Superoxide anion radical (O2•−). J Biol Chem 1997; 272: 18515–18517.
Faraci FM, Didion SP . Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 2004; 24: 1367–1373.
Son SM . Role of vascular reactive oxygen species in development of vascular abnormalities in diabetes. Diabetes Res Clin Pract 2007; 77 (Suppl 1): S65–S70.
Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE . Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 2005; 280: 39616–39626.
Kitada M, Koya D, Sugimoto T, Isono M, Araki S, Kashiwagi A, Haneda M . Translocation of glomerular p47phox and p67phox by protein kinase C-beta activation is required for oxidative stress in diabetic nephropathy. Diabetes 2003; 52: 2603–2614.
Fujita H, Fujishima H, Chida S, Takahashi K, Qi Z, Kanetsuna Y, Breyer MD, Harris RC, Yamada Y, Takahashi T . Reduction of renal superoxide dismutase in progressive diabetic nephropathy. J Am Soc Nephrol 2009; 20: 1303–1313.
Ram CV . Angiotensin receptor blockers: current status and future prospects. Am J Med 2008; 121: 656–663.
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860.
Viberti G, Wheeldon NM . Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 2002; 106: 672–678.
Ogawa S, Mori T, Nako K, Kato T, Takeuchi K, Ito S . Angiotensin II type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathy. Hypertension 2006; 47: 699–705.
Nakamura T, Fujiwara N, Sato E, Ueda Y, Sugaya T, Koide H . Renoprotective effects of various angiotensin II receptor blockers in patients with early-Stage diabetic nephropathy. Kidney Blood Press Res 2010; 33: 213–220.
Brownlee M . The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615–1625.
Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG . Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 1996; 97: 1916–1923.
Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW . Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994; 74: 1141–1148.
Fujimoto S, Satoh M, Horike H, Hatta H, Haruna Y, Kobayashi S, Namikoshi T, Arakawa S, Tomita N, Kashihara N . Olmesartan ameliorates progressive glomerular injury in subtotal nephrectomized rats through suppression of superoxide production. Hypertens Res 2008; 31: 305–313.
Sugiyama H, Kobayashi M, Wang DH, Sunami R, Maeshima Y, Yamasaki Y, Masuoka N, Kira S, Makino H . Telmisartan inhibits both oxidative stress and renal fibrosis after unilateral ureteral obstruction in acatalasemic mice. Nephrol Dial Transplant 2005; 20: 2670–2680.
Negi G, Kumar A, Joshi RP, Sharma SS . Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun 2011; 408: 1–5.
Chan K, Kan YW . Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci USA 1999; 96: 12731–12736.
Chan K, Han XD, Kan YW . An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA 2001; 98: 4611–4616.
Schluter T, Steinbach AC, Steffen A, Rettig R, Grisk O . Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res 2008; 80: 271–279.
Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD . Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 2005; 54: 2628–2637.
Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD . Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 2004; 286: F590–F596.
Fujita H, Kakei M, Fujishima H, Morii T, Yamada Y, Qi Z, Breyer MD . Effect of selective cyclooxygenase-2 (COX-2) inhibitor treatment on glucose-stimulated insulin secretion in C57BL/6 mice. Biochem Biophys Res Commun 2007; 363: 37–43.
Takaya T, Kawashima S, Shinohara M, Yamashita T, Toh R, Sasaki N, Inoue N, Hirata K, Yokoyama M . Angiotensin II type 1 receptor blocker telmisartan suppresses superoxide production and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. Atherosclerosis 2006; 186: 402–410.
Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW . Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension 2004; 43: 993–1002.
Yoshioka M, Kayo T, Ikeda T, Koizumi A . A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997; 46: 887–894.
Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T . A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 1999; 103: 27–37.
Szkudelski T . The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001; 50: 537–546.
Uzu T, Sawaguchi M, Maegawa H, Kashiwagi A . Impact of renin-angiotensin system inhibition on microalbuminuria in type 2 diabetes: a post hoc analysis of the Shiga Microalbuminuria Reduction Trial (SMART). Hypertens Res 2008; 31: 1171–1176.
Liu F, Wei CC, Wu SJ, Chenier I, Zhang SL, Filep JG, Ingelfinger JR, Chan JS . Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int 2009; 75: 156–166.
Burnier M . Telmisartan: a different angiotensin II receptor blocker protecting a different population? J Int Med Res 2009; 37: 1662–1679.
Kakuta H, Sudoh K, Sasamata M, Yamagishi S . Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockers. Int J Clin Pharmacol Res 2005; 25: 41–46.
Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL . Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension 2004; 43: 48–56.
Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, Matsumura T, Tokunaga H, Brownlee M, Araki E . Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes 2003; 52: 2570–2577.
Ditzel J, Schwartz M . Abnormally increased glomerular filtration rate in short-term insulin-treated diabetic subjects. Diabetes 1967; 16: 264–267.
Hostetter TH, Troy JL, Brenner BM . Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int 1981; 19: 410–415.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (No. 20590943 and No. 23591177, to H Fujita) from the Ministry of Education, Science and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Fujita, H., Fujishima, H., Morii, T. et al. Modulation of renal superoxide dismutase by telmisartan therapy in C57BL/6-Ins2Akita diabetic mice. Hypertens Res 35, 213–220 (2012). https://doi.org/10.1038/hr.2011.176
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2011.176
Keywords
This article is cited by
-
Clinical and preclinical evidence that angiotensin-converting enzyme inhibitors and angiotensin receptor blockers prevent diabetic peripheral neuropathy
Scientific Reports (2024)
-
Vitamin D improves diabetic nephropathy in rats by inhibiting renin and relieving oxidative stress
Journal of Endocrinological Investigation (2016)
-
Angiotensin(1–7) attenuates the progression of streptozotocin-induced diabetic renal injury better than angiotensin receptor blockade
Kidney International (2015)
-
The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential
Kidney International (2014)
-
Deletion of p47 phox attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouse
Diabetologia (2012)