Abstract
Chronic low blood pressure is typically accompanied with symptoms such as fatigue, reduced drive, dizziness, headaches and cold limbs. Alterations in autonomic cardiovascular regulation are assumed to be involved in the etiology of this condition, that is, increased baroreflex sensitivity (BRS) as well as reduced sympathetic and augmented parasympathetic influences on cardiovascular regulation. In this study, the acute effects of the blood pressure-enhancing alpha-adrenergic agonist, midodrine, on autonomic cardiovascular control were investigated in 50 hypotensive persons (mean blood pressure 96/61 mm Hg) based on a placebo-controlled double blind design. BRS was determined using sequence analysis. Spectral analysis of heart rate variability was carried out to quantify sympathetic and parasympathetic cardiac control. Parameters were obtained at rest and during mental stress. Drug application led to marked increases in blood pressure, BRS and respiratory sinus arrhythmia (RSA), whereas heart rate and power in the low frequency (LF) band of the heart rate variability spectrum decreased. The augmentation of RSA and reduction of LF power indicate a shift of the sympathovagal balance toward increased parasympathetic and reduced sympathetic influences on heart rate. Like the increase in BRS, these modulations represent a counter-regulatory autonomic response to the blood pressure elevation, which is initiated to return blood pressure to the initial value or to an individual set point. The finding challenges the use of α-sympathomimetics in the treatment of chronic hypotension as they may not reduce but may instead exacerbate the autonomic dysbalance related to this condition.
Similar content being viewed by others
Introduction
Chronic low blood pressure is typically accompanied with symptoms such as fatigue, reduced drive, dizziness, headaches and cold limbs, which can have a considerable impact on subjective well being and quality of life.1, 2 Reduced cognitive performance, as well as diminished cerebral blood flow and cortical activation have been documented in this condition.3, 4, 5 According to the World Health Organization (WHO),6 hypotension is to be diagnosed when systolic blood pressure falls below 100 mm Hg in women and 110 mm Hg in men. The condition of chronic (‘essential’) hypotension must be distinguished from secondary hypotension, caused, for example, by blood loss or medication, as well as from the orthostatic form, that is, circulatory problems when assuming an upright position.7
It has been considered whether alterations in autonomic cardiovascular regulation are involved in the etiology of chronic hypotension.5, 8 Duschek et al.9 reported increased baroreflex sensitivity (BRS) in a hypotensive sample. The baroreflex consists of a negative feedback loop in which changes in the firing rate of the arterial baroreceptors lead to compensatory alterations in heart rate and cardiac contractility.10 Current research suggests that the baroreflex contributes not only to the buffering of transient changes in blood pressure, but also to its long-term setting.11, 12 This is supported by the observation that BRS is reduced in chronically elevated blood pressure, which is thought to have a function in the genesis of this condition.13 In normotensive participants, BRS and tonic blood pressure are inversely related.12 In a recent longitudinal study, reduced BRS predicted a five-year rise in blood pressure.14 Furthermore, it has been shown that the increase of BRS, by means of biofeedback, is followed by a reduction in blood pressure.15 While the operating point of the baroreflex is believed to be increased in individuals with elevated blood pressure, resetting to low blood pressure values may occur in persons with hypotension.9 Besides aberrant baroreflex function, reduced sympathetic and increased parasympathetic influences on cardiovascular regulation have also been postulated in chronic hypotension.5, 16 Several studies have documented generally diminished sympathetic tone,17, 18 and Cadalbert16 reported augmented respiratory sinus arrhythmia (RSA) in chronically hypotensive participants, indicating increased vagal influences on heart rate.
Sympathomimetic drugs have an important function in the treatment of hypotensive disorders. The α-adrenergic agent, midodrine, for instance, is well known to reduce orthostatic symptoms.19, 20 In participants with chronic low blood pressure, midodrine yielded positive hemodynamic effects in terms of raising blood pressure, vascular resistance and stroke volume.21 Furthermore, it proved effective in increasing both cerebral blood flow and mental performance in this population.22 Knowledge about possible effects of α-adrenergic treatment on autonomic cardiovascular regulation, however, remains sparse. This seems to be of particular interest given the likely involvement of these control mechanisms in the etiology of chronic hypotension.
This placebo-controlled double blind study investigated the effects of a single-dose application of midodrine on baroreflex function, as well as on sympathetic and parasympathetic cardiac control in chronic hypotension. A decrease in BRS, as well as increased sympathetic and decreased parasympathetic cardiac influences would imply a reduction in the dysbalance of autonomic cardiovascular regulation. BRS was quantified by analyzing the spontaneous co-variation of systolic blood pressure and heart cycle duration (‘sequence analysis’).23, 24 In addition, spectral analysis of heart rate variability was carried out.25 RSA, expressed by spectral power in the high frequency (HF) band, was applied as an index of vagal control of heart rate.25, 26 Spectral power in the low frequency (LF) band was also obtained. While LF power was formerly viewed as a pure sympathetic index, more recent research indicates that it represents both sympathetic and parasympathetic cardiac influences.25, 26 As an index of sympathovagal balance the LF/HF ratio was used.27, 28, 29 To get a more comprehensive picture, all parameters were obtained during resting conditions as well as under mental stress. For the latter purpose, a mental arithmetic task was applied.
Methods
Participants
The study sample comprised 50 chronic hypotensive participants according to the WHO definition.6 None of them suffered from a relevant physical disease or mental disorder. Health status was assessed by means of an anamnestic interview and a questionnaire covering diseases of the cardiovascular, respiratory, gastrointestinal and urogenital systems, the thyroid, the liver, as well as metabolic diseases and psychiatric disorders. None of the participants used any kind of medication affecting the cardiovascular or central/peripheral nervous system. In addition, participants using medication with known interactions with midodrine were excluded from participation.
Participants were randomly assigned to one of two study groups administered with either midodrine or placebo. Both groups included 18 women and 7 men. Information regarding blood pressure and heart rate at the beginning of the experimental session as well as age and body mass index are given in Table 1. Nine women in the midodrine-treated and seven in the placebo group were using oral contraceptives. Forty of the participants were university students (20 in each group). Five of the remaining participants were employees, three were self-employed and two were unemployed.
Recording of hemodynamic data
Blood pressure and electrocardiogram were monitored continuously (Finometer Model-2, Finapres Medical Systems, Amsterdam, The Netherlands). The blood pressure cuff of the device was applied to the mid-phalanx of the second finger of the right hand. To control for the influence of hydrostatic level errors, the height correction unit integrated in the system was used. For periodic recalibration, the device's physiocal feature30 was in operation. For electrocardiogram recording, two active electrodes (Ag/AgCl) were placed at the right mid-clavicle and at the lowest left rib. The back of the left hand was grounded. Recordings were conducted at a sample rate of 200 Hz.
Substance administration
Dosage of midodrine (trade name in Germany: gutron) was 0.4 mg per kg body weight. The placebo consisted of 4.8 mg of alcohol per kg body weight, equivalent to the alcohol content of gutron. Both the drug and the placebo were dispensed in a glass of water. The group assignment and substance administration were carried out by an assistant who was not otherwise involved in the experiment. Both the participants and the experimenter were blind to the respective conditions.
Procedure
The study was approved by a local ethics commission, and participants gave informed consent before the experiment began. To determine hypotension, blood pressure was taken during screening sessions, which were conducted, at least, one week before the main experiment, and once more at the beginning of the experimental session. For this purpose, after a resting period of 10 min, three blood pressure measurements were taken with 5 min rest intervals in between. Using an automatic inflation monitor (MIT, TYP M CR15; Omron, Bannockburn, IL, USA; validated according to Association for the Advancement of Medical Instrumentation (AAMI) and British Hypertension Society (BHS) international protocols), sphygmomanometrical measurements were taken at the upper arm with the participant in a seated position. Women with a mean systolic blood pressure below 100 mm Hg and men with a mean value below 110 mm Hg were included. The criterion had to be fulfilled at both the screening and experimental sessions.
Hemodynamic indices were obtained at rest and during mental stress induced by a serial subtraction task. During the 5 min resting phase participants were asked to sit still, to not speak and to relax with their eyes open. The subtraction task consisted of a 3 min interval during which participants had to count down from 700, subtracting 17 each time and saying the numbers out loud. They were asked to carry out the task as quickly and as accurately as possible.
As the maximum effect of orally applied midodrine develops after approximately 1 h, the administration was followed by a 60-min break. Thereafter, hemodynamic assessment was repeated. Participants were requested not to smoke or drink either alcohol or beverages containing caffeine for 3 h before the screening and experimental sessions.
Data analysis
In the first step of data processing, the continuously recorded blood pressure and electrocardiogram data were re-sampled at 1000 Hz by means of spline interpolation using MATLAB software (The MathWorks, Natick, MA, USA). BRS was quantified based on the blood pressure recordings using a program developed by Reyes del Paso.31 The program locates sequences of 3–6 consecutive heart cycles in which systolic blood pressure increases are accompanied by increases in heart cycle duration (‘up sequences’), and those in which blood pressure decreases are accompanied by decreases in heart cycle duration (‘down sequences’). Each systolic value was paired with the duration of the cycle immediately following it. The minimum changes to be registered in blood pressure and heart cycle duration were fixed at, respectively, 1 mm Hg and 2 ms. For the quantification of BRS, the interval between systolic points (‘intersystolic interval’) was taken as an index of heart cycle duration. BRS was expressed as the change in heart cycle duration (in ms) per mm Hg blood pressure change within these sequences, measured by the slope of the regression line. The values for BRS were computed separately for up and down sequences, as well as for all detected reflex sequences. High correlations between the values for the up and down sequences were found (r between 0.64 and 0.98 for the respective conditions, all P<0.01), and thus further analysis was restricted to the values derived from the sequences in their entirety.
The electrocardiogram raw signal was processed using the software AcqKnowledge 3.8.1 (Biopac Systems, Goleta, CA, USA) that allows R-spike detection and the quantification of R–R intervals. Beat-by-beat values were edited for artifacts, replacing the respective values by linear interpolation. The time series of R–R intervals were subjected to fast Fourier transformation. For this purpose, software, which follows the Task Force guidelines,32 and was designed by the ‘Biosignal Analysis and Medical Imaging Group’,33 was used. RSA was indexed by spectral power density in the frequency range of 0.15–0.40 Hz (HF band). According to recent suggestions,26 respiratory rate was not controlled for in the computation of RSA. The LF band was defined as spectral power density in the frequency range of 0.04–0.15 Hz. LF and HF power are reported in normalized units, which represent the relative power of each component in proportion to the total power of the extracted spectrum.32 The LF/HF ratio was also calculated.
All indices were obtained for the time intervals of the resting and mental stress conditions. Statistical analysis comprised analysis of variance procedures with the experimental group (midodrine vs. placebo) as a between-participants factor and point of measurement (before vs. after substance administration) and experimental condition (rest vs. mental stress) as within-participants factors. Dependent variables comprised BRS, HF power (RSA), LF power, LF/HF ratio, systolic blood pressure and R–R interval. To ensure the reliability of the quantification of BRS, the number of baroreflex sequences per minute was also determined for each of the conditions and was included in the analysis. In addition to the analysis of variance procedures, changes between points of measurement in all dependent variables in either experimental group were checked for significance using post-hoc t-tests.
Results
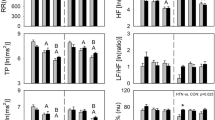
The midodrine and the placebo groups did not differ in any of the dependent variables at the pre-test stage (all P>0.05). Figure 1 shows the changes in BRS between both points of measurement. BRS increased in the midodrine-treated group and changed only slightly under placebo. This held true for both experimental conditions, that is, rest and mental stress. The analysis of variance showed a significant interaction between point of measurement and experimental group (F[1, 48]=24.7, P<0.01). BRS was overall lower during mental stress than at rest, which is confirmed by a significant main effect of experimental condition (F[1, 48]=30.6, P<0.01). The number of baroreflex sequences detected per minute decreased following midodrine administration (interaction point of measurement by group: F[1, 48]=28.3, P<0.01). This held true for the resting phase (midodrine: pre-drug M=11.7, s.d.=4.2; post-drug M=4.0, s.d.=3.1; placebo: pre-drug M=11.1, s.d.=3.8; post-drug M=9.2, s.d.=4.7), as well as the stress condition (midodrine: pre-drug M=10.7, s.d.=2.3; post-drug M=8.0, s.d.=2.6; placebo: pre-drug M=10.6, s.d.=3.6; post-drug M=10.1, s.d.=3.8). (The reduction in the numbers of baroreflex sequences was most likely due to the reduced heart rate caused by midodrine, and the lower number of cardiac cycles, therefore, available for sequence analysis. Nonetheless, given the durations of the periods of rest (5 min) and mental stress (3 min), the detected sequences provided a sufficient base for the reliable quantification of BRS.)
The results of heart rate variability analysis are given in Figure 2. RSA, indexed by HF power, increased under midodrine, and LF power decreased. Again, the placebo group experienced relatively minor changes. The interaction between point of measurement and experimental group was significant for both HF and LF power (HF power: F[1, 48]=22.9, P<0.01; LF power: F[1, 48]=22.9, P<0.01). When compared with levels during rest, RSA was significantly lower during stress (condition: F[1, 48]=41.2, P<0.01), and HF power was significantly higher (condition: F[1, 48]=41.2, P<0.01). The LF/HF ratio decreased between points of measurement in the midodrine-treated group. In the placebo group, a slight increase between points of measurement was observed for the resting, and a slight decrease for the stress conditions. The interaction between point of measurement and experimental group was significant (F[1, 48]=11.1, P<0.01). The LF/HF ratio was overall significantly higher during stress than at rest (condition: F[1, 48]=15.6, P<0.01).
Systolic blood pressure and R–R interval increased under midodrine and remained virtually unchanged under placebo (Figure 3). For both parameters, the interaction between point of measurement and experimental group was significant (systolic blood pressure: F[1, 48]=17.1, P<0.01; R–R interval: F[1, 48]=86.8, P<0.01). When compared with levels during rest, systolic blood pressure was significantly higher (condition: F[1, 48]=404.6, P<0.01), and R–R interval was significantly lower during mental stress (condition: F[1, 48]=114.1, P<0.01).
Results of the post hoc t-tests are given in Table 2. In the midodrine-treated group, all parameters changed significantly between points of measurement. This held true for the parameters obtained at rest, as well as, during stress. In contrast, none of the changes reached significance in the placebo group.
Discussion
The study showed a marked increase in the sensitivity of the cardiac baroreflex following administration of the vasopressor agent, midodrine, in participants with chronic low blood pressure. Heart rate variability increased in the high- and decreased in the low-frequency range. While the increase in HF power clearly indicates drug-induced enhancement of parasympathetic cardiac tone, the reduction in LF power cannot be interpreted without ambiguity, given that it represents both sympathetic and parasympathetic influences.25 However, considering the rise in RSA, it seems likely that the reduction in LF power was not due to decreased parasympathetic influences but instead due to decreased sympathetic influences in the control of heart rate. Taken together, the findings suggest that the previously described increased BRS and dominance of the parasympathetic system in cardiac regulation of chronic hypotension5, 9, 16 were further augmented by drug application.
Midodrine does not cross the blood–brain barrier and, therefore, does not directly influence central nervous control of the cardiovascular system.19, 34 Hence, the autonomic modulations may be ascribed to the hemodynamic effects of the drug. As an α-receptor agonist, midodrine induces arterial and venous vasoconstriction leading to increased peripheral resistance, which, in turn, causes blood pressure elevation.19, 21 In this state, counter-regulatory processes are initiated to return blood pressure to the initial value or to an individual set point. The observed sympathetic inhibition and parasympathetic activation may be regarded as parts of this compensatory mechanism.
The baroreflex is crucial for the autonomic counter-regulation. The compensatory heart rate adjustment triggered by changing arterial pressure is almost exclusively transmitted by the parasympathetic system.10, 35 Therefore, the rise in RSA following pharmacological blood pressure elevation may largely be considered to be a result of baroreflex activation. The role of the reflex, as a source of parasympathetic control of heart rate, is underlined, for instance, by a study by Reyes del Paso et al.36 In this study, a pharmacological vagal blockade reduced BRS to values close to zero. After β-adrenergic blockade, during which the heart rate is predominantly under parasympathetic control, BRS accounted for more than 90% of heart rate variance. On a neuroanatomical level, this issue is reflected by structural interactions between the brain stem units subserving the baroreflex system. The first synapse of the baroreceptor afferents is located in the nucleus of the solitary tract, which gives rise to projections to the nucleus ambiguus and the dorsal motor nucleus. The latter nuclei constitute the starting points of vagal efferents, through which the heart rate response is executed.37, 38
In chronic hypotension, the operating point of the baroreflex is believed to be reduced to lower blood pressure levels.9 This study suggests that pharmacological blood pressure enhancement does not entail the resetting of the operating point to higher values, which could, for instance result from baroreceptor habituation.38 Instead, the rise in BRS suggests that the attempt of the system to decrease blood pressure to low set points may even be strengthened by the application of an adrenergic drug.
The augmentation of blood pressure, LF power and LF/HF ratio, as well as the decrease in R–R interval, RSA and BRS during mental stress are consistent with previous research.37 The pattern of changes in the heart rate variability spectrum reflects task-related sympathetic activation and vagal withdrawal. The decrease in BRS attenuates the buffering effect of the reflex, thereby facilitating stress-induced blood pressure elevation.9, 39 These modulations in autonomic control may be regarded as adaptive mechanisms enabling the improvement of energetic and metabolic supply during acute stress.40
Finally, the results may have clinical implications. Blood pressure can be enhanced efficiently by α-adrenergic treatment in chronic hypotension. This is, however, accompanied by strong autonomic counter-regulation in terms of a shift of sympathovagal balance toward increased parasympathetic and reduced sympathetic influences in cardiac control. This implies that the magnitude of the autonomic dysbalance, which is regarded as relevant in the etiology of chronic hypotension,5, 9 is not reduced, but actually increased. The pronounced reduction in heart rate may constitute another problem. As previously reported, midodrine application leads to a marked increase in stroke volume and total peripheral resistance, but, due to heart deceleration, it does not cause an increase in cardiac output.21 Therefore, the drug's efficiency in improving organ perfusion is possibly less than ideal. One should, however, not overlook the fact that the effects of a single application of the drug observed in this study may not be generalized to clinical conditions. In case of long-term application, different autonomic effects, for instance, as a result of more pronounced baroreceptor habituation, cannot be ruled out. For this reason further clinical trials are certainly worthwhile.
References
Rosengren A, Tibblin G, Wilhelmsen L . Low systolic blood pressure and self perceived wellbeing in middle aged men. Br Med J 1993; 306: 243–246.
Wessely S, Nickson J, Cox B . Symptoms of low blood pressure: a population study. Br Med J 1990; 301: 362–365.
Duschek S, Schandry R . Cognitive performance and cerebral blood flow in essential hypotension. Psychophysiology 2004; 41: 905–913.
Duschek S, Meinhardt J, Schandry R . Reduced cortical activity due to chronic low blood pressure: an EEG study. Biol Psychol 2006; 72: 241–250.
Duschek S, Schandry R . Reduced brain perfusion and cognitive performance due to essential hypotension. Clin Auton Res 2007; 17: 69–76.
WHO. Arterial Hypertension: Technical REPORT Series No. 628. World Health Organisation: Genova, 1978.
Low PA, Singer W . Management of neurogenic orthostatic hypotension: an update. Lancet Neurol 2008; 7: 451–458.
Weisz N, Schandry R, Jacobs A, Mialet J, Duschek S . Early contingent negative variation of the EEG and attentional flexibility are reduced in hypotension. Int J Psychophysiol 2002; 45 (suppl 3): 253–260.
Duschek S, Dietel A, Schandry R, Reyes del Paso GA . Increased baroreflex sensitivity and reduced cardiovascular reactivity in chronic low blood pressure. Hypertens Res 2008a; 31: 1873–1878.
Levy MN, Pappano AJ . Cardiovascular Physiology. Mosby Elsevier: Philadelphia, 2007.
Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS . Prolonged activation of the baroreflex produces sustained hypotension. Hypertension 2004; 43: 306–311.
Hesse C, Charkoudian N, Liu Z, Joyner MJ, Eisenach JH . Baroreflex sensitivity inversely correlates with ambulatory blood pressure in healthy normotensive humans. Hypertension 2007; 50: 41–46.
Biaggioni I . Sympathetic control of the circulation in hypertension: lessons from autonomic disorders. Curr Opin Nephrol Hypertens 2003; 12: 175–180.
Ducher M, Fauvel JP, Cerrutti C . Risk profile in hypertension genesis: a five-year follow-up study. Am J Hypertens 2006; 19: 775–781.
Reyes del Paso GA, González MI . Modification of baroreceptor cardiac reflex function by biofeedback. Appl Psychophysiol Biofeedback 2004; 29: 197–211.
Cadalbert B . Die Psychophysiologie des niedrigen Blutdrucks: Kreislaufregulation, Lebensgewohnheiten und Beschwerden [The psychophysiology of low blood pressure: Cardiovascular regulation, lifestyle and complaints]. Peter Lang, Frankfurt a.M., 1997.
Richter-Heinrich E, Borys M, Sprung H, Läuter J . Psychophysiologische Reaktionsprofile von Hypo- und Hypertonikern. Dtsch Gesundheitsw 1971; 32: 1481–1489.
Fredrikson M, Edmann G, Levander SE, Schalling D, Svensson J, Tuomisto M . Electrodermal responsivity in young hypotensive and hypertensive men. Psychophysiology 1990; 27: 649–655.
McTavish D, Goa KL . Midodrine. A review of its pharmacological properties and therapeutic use in orthostatic hypotension and secondary hypotensive disorders. Drugs 1989; 38: 757–777.
Wright RA, Kaufmann HC, Perera R, Opfer-Gehrking TL, McElligott MA, Sheng KN, Low PA . A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology 1988; 51: 120–124.
Duschek S, Heiss H, Buechner B, Werner NS, Schandry R, Reyes del Paso GA . Hemodynamic determinants of chronic hypotension and their modification through vasopressor application. J Physiol Sci 2009; 59: 105–109.
Duschek S, Hadjamu M, Schandry R . Enhancement of cerebral blood flow and cognitive performance due to pharmacological blood pressure elevation in chronic hypotension. Psychophysiology 2007; 44: 145–153.
Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G . Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol 1988; 254: H377–H383.
Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G . Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension 1988; 12: 214–222.
Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW . Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 1997; 34: 623–648.
Denver JW, Reed SF, Porges SW . Methodological issues in the quantification of respiratory sinus arrhythmia. Biol Psychol 2007; 74: 286–294.
Malliani A, Pagani M, Lombardi F, Cerutti S . Cardiovascular neural regulation explored in the frequency domain. Circulation 1991; 84: 482–492.
Malliani A, Pagani M, Lombardi F . Physiology and clinical implications of variability of cardiovascular parameters with focus on heart rate and blood pressure. Am J Cardiol 1994; 73: 3C–9C.
Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A . Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994; 90: 1826–1831.
Wesseling KH, De Wit B, Van der Hoeven GMA, Van Goudoever J, Settels JJ . Physiocal, calibrating finger vascular physiology for Finapres. Homeostasis 1995; 36: 67–82.
Reyes del Paso GA . A program to assess baroreceptor cardiac reflex function. Behav Res Meth Instrum 1994; 26: 62–64.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93: 1043–1065.
Niskanen JP, Tarvainen MP, Ranta-aho PO, Karjalainen PA . Software for advanced HRV analysis. Comp Meth Prog Bio 2004; 76: 73–81.
Kaufmann H, Brannan T, Krakoff L, Yahr MD, Mandeli J . Treatment of orthostatic hypotension due to autonomic failure with a peripheral alpha-adrenergic agonist (midodrine). Neurology 1988; 38: 951–956.
Sleight P, La Rovere MT, Mortara A, Pinna GD, Maestri R, Leuzzi S, Bianchini B, Tavazzi L, Bernardi L . Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin Sci (Lond) 1995; 88: 103–109.
Reyes del Paso GA, Langewitz W, Robles H, Perez N . A between-subjects comparison of respiratory sinus arrythmia and baroreceptor cardiac reflex sensitivity as non-invasive measures of tonic parasympathetic cardiac control. Int J Psychophysiol 1996; 22: 163–171.
van Roon AM, Mulder LJM, Althaus M, Mulder G . Introducing a baroreflex model for studying cardiovascular effects of mental workload. Psychophysiology 2004; 41: 961–981.
Rau H, Elbert T . Psychophysiology of arterial baroreceptors and the etiology of hypertension. Biol Psychol 2001; 57: 179–201.
Duschek S, Werner N, Kapan N, Reyes del Paso GA . Patterns of cerebral blood flow and systemic hemodynamics during arithmetic processing. J Psychophysiol 2008; 22: 81–90.
Pinel JPJ . Biopsychology. Pearson Education, Boston, 2007.
Acknowledgements
We thank Boriana Buechner for medical assistance, and Ann-Kristin Adam and Nina Fricke for their help with subject acquisition. We also thank Annina Neumann and Maria Martín for the great help with the analysis of the hemodynamic data. This study was supported by funds from by the German Research Foundation (Project-No.: DU 447/1-1). The cooperation between the Universities of Munich and Jaén was supported by the German Academic Exchange Service and the Spanish Ministry of Education and Science (Project-No.: D/07/13368).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duschek, S., Heiss, H., Werner, N. et al. Modulations of autonomic cardiovascular control following acute alpha-adrenergic treatment in chronic hypotension. Hypertens Res 32, 938–943 (2009). https://doi.org/10.1038/hr.2009.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2009.115
Keywords
This article is cited by
-
Autonomic Cardiovascular Control and Executive Function in Chronic Hypotension
Annals of Behavioral Medicine (2017)
-
Chronic hypotension and modulation of autonomic cardiovascular regulation
Hypertension Research (2009)