Abstract
Mutations in the bone morphogenetic protein receptor type II (BMPR2) gene may result in the development of pulmonary arterial hypertension (PAH). However, the contribution of disease-causing mutations to the disease characteristics and responsiveness to recent treatment remains to be elucidated. We report three Japanese cases of advanced PAH with novel BMPR2 mutations, including two splicing mutations (IVS8-6_7delTTinsA and IVS9-2A>G) and one deletion (c.1279delG) mutation.
Similar content being viewed by others
Pulmonary arterial hypertension (PAH) is characterized by the progressive proliferative vasculopathy of small pulmonary arteries resulting in increased pulmonary vascular resistance (PVR) and right ventricular failure. In ~75% of the cases of heritable PAH (World Health Organization (WHO) group 1), mutations in the gene encoding bone morphogenetic protein receptor type II (BMPR2) have been observed.1 BMPR2 signaling-related genes, such as ACVRL1 (also known as ALK1) and ENG, are also known causative genes.2–4 The role of BMPR2 signaling in the maintenance of the pulmonary vasculature has been verified using several animal models.5–8
BMPR2 is a 190-kb gene with 13 exons that encodes type II transforming growth factor-β (TGF-β) serine/threonine kinase receptor (BMPR2). Until recently, more than 450 mutations have been identified in BMPR2, and these mutations are spread throughout the gene.9,10 PAH patients with BMPR2 mutations have higher disease activity than those without BMPR2 mutations;11 however, a recent combination therapy with an endothelin receptor antagonist, a phosphodiesterase type 5 (PDE5) inhibitor, and a prostaglandin I2 (PGI2) analog was able to improve the prognosis of certain patients with idiopathic PAH.12 Thus, further research is warranted to clarify the relationship between BMPR2 mutations and disease characteristics.
Here, we report three novel mutations, including two splicing mutations (IVS8-6_7delTTinsA and IVS9-2A>G) and one deletion (c.1279delG) mutation, in the intracellular serine/threonine kinase domain of BMPR2 that were associated with advanced PAH in Japanese patients who were effectively treated with the triple combinatorial therapy.
The diagnosis of idiopathic and heritable PAH was based on the measurements of mean pulmonary artery pressure (mPAP) ⩾25 mm Hg at rest, pulmonary artery wedge pressure (PAWP) ⩽15 mm Hg and PVR>3 Wood units at right heart catheterization (RHC), and the absence of any secondary causes of elevated pulmonary arterial pressure.13,14 The genetic analysis of PAH patients was approved by the Ethics Committee at the University of Tokyo Hospital (G10013). Genomic DNA was extracted from the peripheral blood. The mutation analysis of PAH-related genes (BMPR2, ACVRL1, and ENG) was performed using the Sanger sequencing method as previously described.15 All primer sequences are available upon request. Total RNA was extracted from white blood cells using an RNeasy Mini Kit (Qiagen, Hilden, Germany). First-strand complimentary DNA (cDNA) synthesis was performed using 500 ng of total RNA, random hexamers, and ReverTra Ace (Toyobo, Osaka, Japan). To analyze the splicing patterns, PCR was performed using primers located in exons 8 and 11: forward, 5′- cccatcgagatttaaacagcaga-3′ and reverse, 5′- tgactgttgggctcacagat-3′, respectively. The wild-type PCR product was 567 bp, and the PCR products from the exon 9 and 10 deletion mutants were 419 and 430 bp, respectively. The products were then subcloned into a pMD20T vector using a Mighty TA-cloning Kit (TAKARA, Shiga, Japan) and sequenced for confirmation.
Proband #165 experienced shortness of breath upon exertion at 31 years of age. Echocardiography revealed right ventricular dilatation and septal flattening, and RHC confirmed the presence of pulmonary hypertension with an mPAP of 50 mm Hg. Her mother had died of idiopathic PAH at the age of 34 years; thus, proband #165 was diagnosed with heritable PAH. Acute pulmonary vasoreactivity testing with inhaled nitric oxide (NO) at a concentration of 20 ppm for 10 min was negative, and she was treated with the combination therapy bosentan/macitentan, tadalafil, and beraprost/treprostinil for over 2 years, resulting in a reduction in mPAP to 32 mm Hg (Figure 1).
Japanese cases of PAH. (a) Pedigrees of the Japanese cases with clinical features of PAH. The age is shown in the upper left corner, and ‘d’ indicates the age at death. (b) Patient clinical characteristics and parameters. Proband #Ph7 received oxygen (7 l/min) and dobutamine (2 μg/kg/min) at the time of the first right heart catheterization. Acute vasoreactivity tests were performed with inhaled nitric oxide. Arrow, proband; Circle, female; Diagonal line, died; Filled, PAH; mPAP, mean pulmonary arterial pressure; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; Square, male.
Proband #Ph7 presented with severe dyspnea and hemoptysis at 27 years of age. She received an infusion of dobutamine and non-invasive positive pressure ventilation for severe cardiorespiratory failure. RHC revealed an elevated mPAP of 92 mm Hg and PAWP of 16 mm Hg. She was treated with the combination therapy macitentan, sildenafil, and i.v. epoprostenol for ~2 months, and the follow-up RHC revealed that the mPAP and PAWP decreased to 35 and 4 mm Hg, respectively, without catecholamine support. Her mother died of idiopathic PAH at the age of 46 years; thus, proband # Ph7 was also diagnosed with heritable PAH (Figure 1).
Proband #687 was a 55-year-old female with no apparent relevant family history. She developed progressive dyspnea at 49 years of age, and RHC revealed an elevated mPAP of 65 mm Hg and a normal PAWP of 8 mm Hg. She was diagnosed with idiopathic PAH, after excluding other possible causes of PAH. She was treated with the combination therapy diltiazem, bosentan, tadalafil/sildenafil, and beraprost/treprostinil for over 5 years, and her mPAP was maintained to below 40 mm Hg (Figure 1).
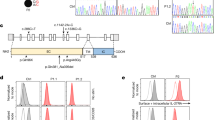
Here, we described three Japanese patients with heritable or idiopathic PAH after the exclusion of other secondary causes of PAH. We performed a genetic analysis for PAH-related genes, including BMPR2, ACVRL1, and ENG, and concluded that intronic mutations, IVS8-6_7delTTinsA (c.1129-6_7delTTinsA) and IVS9-2A>G (c.1277-2A>G), in BMPR2 likely resulted in the PAH phenotypes in probands #165 and #Ph7, respectively. A single-nucleotide deletion (c.1279delG) was concluded to be responsible for PAH in proband #687 (Figure 2a). These variants were absent from public variant databases such as dbSNP, Exome Variant Server, Exome Aggregation Consortium, or Human Genome Variation Browser.
BMPR2 mutation analysis. (a) Genomic DNA sequencing demonstrated intronic mutations in probands #165 and #Ph7 (IVS8-6_7delTTinsA (c.1129-6_7delTTinsA) and IVS9-2A>G (c.1277-2A>G), respectively) and a single-nucleotide deletion in proband #687 (c.1279delG). (b) Splice analysis of exons 9 and 10 using cDNA from white blood cells (left panel). The PCR primers (arrows) were located in exons 8 and 11. The wild-type PCR product was 567 bp, and the PCR products from the exon 9 and 10 deletion mutants were 419 and 430 bp, respectively (right panel). The transcript from probands #165 and #Ph7 had a smaller band; however, two healthy volunteers and proband #687 did not show any apparent extra bands. (c) Direct cDNA sequencing of the wild-type (top) and mutant (bottom) alleles. Exons 9 and 10 were skipped in the mutant transcripts from probands #165 and #Ph7, respectively. In proband #687, the wild-type transcript was 567 bp in length and the mutant transcript was 566 bp in length because one guanine (G) nucleotide was missing at the third position of exon 10. cDNA, complimentary DNA.
The two intronic variants located at acceptor splice sites and one single-nucleotide deletion mutation were located in the intracellular serine/threonine kinase domain of BMPR2. To observe the effects of these mutations, we examined the transcriptional consequences using cDNA generated from the messenger RNA of white blood cells. The amplification of exons 8–11 (567 bp) and the direct sequencing of the corresponding cDNA sequence revealed an out-of-frame transcript missing exon 9 in proband #165 (p.Val377GlyfsX48) and an out-of-frame transcript missing exon 10 in proband #Ph7 (p.Glu427SerfsX26) (Figure 2b and c). These mutant transcripts were much less abundant than the wild-type transcripts.
In proband #687, a direct sequence analysis of the amplified PCR products revealed the existence of two fragments of different sizes; 19 out of 25 clones (76%) were 567 bp in length, corresponding to the wild-type allele, and 6 (24%) were 566 bp in length owing to the loss of one guanine (G) nucleotide at the position 1279 (p.Glu427AsnfsX47) (Figure 2c).
In this study, we identified three cases of advanced PAH in Japanese patients with novel BMPR2 mutations including two splicing mutations (IVS8-6_7delTTinsA and IVS9-2A>G) and one deletion mutation (c.1279delG). We were only able to perform genetic analyses of the surviving affected patients; thus, we could not show cosegregation with the PAH phenotype. However, all three mutations produced premature termination codons in the intracellular serine/threonine kinase domain, which probably caused the protein to be abnormally shortened or destroyed; thus, we concluded that these mutations were the causes of the heritable PAH.
The penetrance for heritable PAH is low, with an estimated lifetime risk of 20–30%.16 PAH may be induced by a combination of additional genetic and/or environmental risk factors that make mutation carriers more susceptible to the disease. Sex, pregnancy, altitude, drugs, or other diseases (e.g., collagen diseases) are reported to be involved in the development of PAH.14 Indeed, female patients are more prone to the development of PAH, and the female/male incidence ratio in PAH patients with BMPR2 mutations is ~2:1.11 In addition, PAH is more prevalent in young women, and pregnancy often triggers the onset and/or progression of the disease.17 In the present cases, the patients were all female, but they did not have a history of pregnancy or other known risk factors.
The BMPR2 serine/threonine kinase receptor contains an extracellular ligand-binding domain, a transmembrane region, a serine/threonine kinase domain, and a cytoplasmic tail, and previously reported BMPR2 mutations are distributed across all these domains.9,10 Recently, Girerd et al.18 reported that patients with mutations in the cytoplasmic tail of BMPR2 exhibit less severe phenotypes and are more likely to respond to acute pulmonary vasoreactivity testing with inhaled NO and long-term therapy with calcium channel blockers. In the cases presented here, the mutations were all located in the intracellular serine/threonine kinase domain, and the disease activities in the patients were high with negative pulmonary vasoreactivity tests.
Recently, a combination therapy with PGI2 analogs, endothelin receptor antagonists and PDE5 inhibitors has been shown to reduce mPAP and hospitalizations of patients who were previously considered difficult to treat, which has led to an improvement in the overall survival.19 Patients with PAH carrying BMPR2 mutations can also receive temporal benefits from the combination therapy;20 however, the daily living abilities (functional performance status) of these patients did not fully recover similar to our present cases. Thus, the relationship between gene mutations and long-term clinical outcomes should be further investigated.
In conclusion, we reported three Japanese cases of advanced PAH with novel BMPR2 mutations. Further analysis is warranted to establish the clinical usefulness of BMPR2 resequencing to clarify the genetic backgrounds in these cases.
References
References
Austin ED, Loyd JE . The genetics of pulmonary arterial hypertension. Circ Res 2014; 115: 189–202.
Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001; 345: 325–334.
Harrison RE, Flanagan JA, Sankelo M, Abdalla SA, Rowell J, Machado RD et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet 2003; 40: 865–871.
Chaouat A, Coulet F, Favre C, Simonneau G, Weitzenblum E, Soubrier F et al. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax 2004; 59: 446–448.
Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM et al. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 2004; 287: L1241–L1247.
Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H et al. Genetic ablation of the Bmpr2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 2008; 118: 722–730.
Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 2013; 123: 3600–3613.
Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 2015; 21: 777–785.
Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S32–S42.
Machado RD, Southgate L, Eichstaedt CA, Aldred MA, Austin ED, Best DH et al. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat 2015; 36: 1113–1127.
Evans JDW, Girerd B, Montani D, Wang XJ, Galiè N, Austin ED et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med 2016; 2600: 1–9.
Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533.
Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–D50.
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41.
Takeda N, Morita H, Fujita D, Inuzuka R, Taniguchi Y, Nawata K et al. A deleterious MYH11 mutation causing familial thoracic aortic dissection. Hum Genome Var 2015; 2: 15028.
Larkin EK, Newman JH, Austin ED, Hemnes AR, Wheeler L, Robbins IM et al. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 892–896.
Limoges M, Langleben D, Fox BD, Shear R, Wieczorek P, Rudski LG et al. Pregnancy as a possible trigger for heritable pulmonary arterial hypertension. Pulm Circ 2016; 6: 381–383.
Girerd B, Coulet F, Jais X, Eyries M, Van Der Bruggen C, De Man F et al. Characteristics of pulmonary arterial hypertension in affected carriers of a mutation located in the cytoplasmic tail of bone morphogenetic protein receptor type 2. Chest 2015; 147: 1385–1394.
Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani H-A et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818.
Sitbon O, Jaïs X, Savale L, Cottin V, Bergot E, Macari EA et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J 2014; 43: 1691–1697.
Data Citations
Takeda, Norifumi HGV Database (2017) http://dx.doi.org/10.6084/m9.figshare.hgv.958
Takeda, Norifumi HGV Database (2017) http://dx.doi.org/10.6084/m9.figshare.hgv.961
Takeda, Norifumi HGV Database (2017) http://dx.doi.org/10.6084/m9.figshare.hgv.964
Acknowledgements
We thank Ms Aiko Mochizuki for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Hara, H., Takeda, N., Morita, H. et al. Three novel BMPR2 mutations associated with advanced pulmonary arterial hypertension. Hum Genome Var 4, 17010 (2017). https://doi.org/10.1038/hgv.2017.10
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/hgv.2017.10
This article is cited by
-
Distinct variants affecting differential splicing of TGFBR1 exon 5 cause either Loeys–Dietz syndrome or multiple self-healing squamous epithelioma
European Journal of Human Genetics (2018)