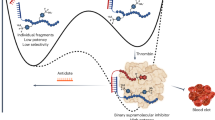
Abstract
Recent hemophilia B clinical trials using adeno-associated virus (AAV) gene delivery have demonstrated much lower coagulation factor IX (FIX) production in patients compared with the high levels observed in animal models and AAV capsid-specific cytotoxic T lymphocyte response elicited at high doses of AAV vectors. These results emphasize the necessity to explore effective approaches for enhancement of AAV transduction. Initially, we found that incubation of all AAV vectors with human serum enhanced AAV transduction. Complementary analytical experiments demonstrated that human serum albumin (HSA) directly interacted with the AAV capsid and augmented AAV transduction. The enhanced transduction was observed with clinical grade HSA. Mechanistic studies suggest that HSA increases AAV binding to target cells, and that the interaction of HSA with AAV does not interfere with the AAV infection pathway. Importantly, HSA incubation during vector dialysis also increased transduction. Finally, HSA enhancement of AAV transduction in a model of hemophilia B displayed greater than a fivefold increase in vector-derived circulating FIX, which improved the bleeding phenotype correction. In conclusion, incubation of HSA with AAV vectors supports a universal augmentation of AAV transduction and, more importantly, this approach can be immediately transitioned to the clinic for the treatment of hemophilia and other diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011; 365: 2357–2365.
Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014; 371: 1994–2004.
Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood 2006; 107: 2653–2661.
Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol Ther 2008; 16: 280–289.
Monahan PE, Sun J, Gui T, Hu G, Hannah WB, Wichlan DG et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum Gene Ther 2015; 26: 69–81.
McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ . Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther 2003; 10: 2112–2118.
McCarty DM, Monahan PE, Samulski RJ . Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 2001; 8: 1248–1254.
Shen S, Horowitz ED, Troupes AN, Brown SM, Pulicherla N, Samulski RJ et al. Engraftment of a galactose receptor footprint onto adeno-associated viral capsids improves transduction efficiency. J Biol Chem 2013; 288: 28814–28823.
Li C, Diprimio N, Bowles DE, Hirsch ML, Monahan PE, Asokan A et al. Single amino acid modification of adeno-associated virus capsid changes transduction and humoral immune profiles. J Virol 2012; 86: 7752–7759.
Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA 2008; 105: 7827–7832.
Mitchell AM, Li C, Samulski RJ . Arsenic trioxide stabilizes accumulations of adeno-associated virus virions at the perinuclear region, increasing transduction in vitro and in vivo. J Virol 2013; 87: 4571–4583.
Mitchell AM, Samulski RJ . Mechanistic insights into the enhancement of adeno-associated virus transduction by proteasome inhibitors. J Virol 2013; 87: 13035–13041.
Douar AM, Poulard K, Stockholm D, Danos O . Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol 2001; 75: 1824–1833.
Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF . Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 2000; 105: 1573–1587.
Ferrari FK, Samulski T, Shenk T, Samulski RJ . Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol 1996; 70: 3227–3234.
Johnson JS, Samulski RJ . Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol 2009; 83: 2632–2644.
Monahan PE, Lothrop CD, Sun J, Hirsch ML, Kafri T, Kantor B et al. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application. Mol Ther 2010; 18: 1907–1916.
Ju XD, Lou SQ, Wang WG, Peng JQ, Tian H . Effect of hydroxyurea and etoposide on transduction of human bone marrow mesenchymal stem and progenitor cell by adeno-associated virus vectors. Acta Pharmacol Sin 2004; 25: 196–202.
Prasad KM, Xu Y, Yang Z, Toufektsian MC, Berr SS, French BA . Topoisomerase inhibition accelerates gene expression after adeno-associated virus-mediated gene transfer to the mammalian heart. Mol Ther 2007; 15: 764–771.
Russell DW, Alexander IE, Miller AD . DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci USA 1995; 92: 5719–5723.
Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U, Engelhardt JF . Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol 2002; 76: 2043–2053.
Wang M, Crosby A, Hastie E, Samulski JJ, McPhee S, Joshua G et al. Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther 2015; 22: 984–992.
Sonntag F, Kother K, Schmidt K, Weghofer M, Raupp C, Nieto K et al. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J Virol 2011; 85: 12686–12697.
Xiao PJ, Li C, Neumann A, Samulski RJ . Quantitative 3D tracing of gene-delivery viral vectors in human cells and animal tissues. Mol Ther 2012; 20: 317–328.
Machida A, Kishimoto S, Ohnuma H, Miyamoto H, Baba K, Oda K et al. A hepatitis B surface antigen polypeptide (P31) with the receptor for polymerized human as well as chimpanzee albumins. Gastroenterology 1983; 85: 268–274.
Pontisso P, Petit MA, Bankowski MJ, Peeples ME . Human liver plasma membranes contain receptors for the hepatitis B virus pre-S1 region and, via polymerized human serum albumin, for the pre-S2 region. J Virol 1989; 63: 1981–1988.
Mehdi H, Kaplan MJ, Anlar FY, Yang X, Bayer R, Sutherland K et al. Hepatitis B virus surface antigen binds to apolipoprotein H. J Virol 1994; 68: 2415–2424.
Andre P, Perlemuter G, Budkowska A, Brechot C, Lotteau V . Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis 2005; 25: 93–104.
Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M Jr et al. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA 2007; 104: 5848–5853.
Chang KS, Jiang J, Cai Z, Luo G . Human apolipoprotein e is required for infectivity and production of hepatitis C virus in cell culture. J Virol 2007; 81: 13783–13793.
Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 2006; 108: 2554–2561.
Jiang H, Wang Z, Serra D, Frank MM, Amalfitano A . Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol Ther 2004; 10: 1140–1142.
Zinn KR, Szalai AJ, Stargel A, Krasnykh V, Chaudhuri TR . Bioluminescence imaging reveals a significant role for complement in liver transduction following intravenous delivery of adenovirus. Gene Ther 2004; 11: 1482–1486.
Denard J, Marolleau B, Jenny C, Rao TN, Fehling HJ, Voit T et al. C-reactive protein (CRP) is essential for efficient systemic transduction of recombinant adeno-associated virus vector 1 (rAAV-1) and rAAV-6 in mice. J Virol 2013; 87: 10784–10791.
Denard J, Beley C, Kotin R, Lai-Kuen R, Blot S, Leh H et al. Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6. J Virol 2012; 86: 6620–6631.
Zaiss AK, Cotter MJ, White LR, Clark SA, Wong NC, Holers VM et al. Complement is an essential component of the immune response to adeno-associated virus vectors. J Virol 2008; 82: 2727–2740.
Pulicherla N, Shen S, Yadav S, Debbink K, Govindasamy L, Agbandje-McKenna M et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol Ther 2011; 19: 1070–1078.
Asokan A, Conway JC, Phillips JL, Li C, Hegge J, Sinnott R et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat Biotechnol 2010; 28: 79–82.
Sleep D . Albumin and its application in drug delivery. Expert Opin Drug Delivery 2015; 12: 793–812.
Fiume L, Manerba M, Di Stefano G . Albumin-drug conjugates in the treatment of hepatic disorders. Expert Opin Drug Delivery 2014; 11: 1203–1217.
Kratz F . Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release 2008; 132: 171–183.
Sand KM, Bern M, Nilsen J, Noordzij HT, Sandlie I, Andersen JT . Unraveling the interaction between FcRn and albumin: opportunities for design of albumin-based therapeutics. Front Immunol 2014; 5: 682.
Elzoghby AO, Samy WM, Elgindy NA . Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release 2012; 157: 168–182.
Elsadek B, Kratz F . Impact of albumin on drug delivery—new applications on the horizon. J Control Release 2012; 157: 4–28.
Bern M, Sand KM, Nilsen J, Sandlie I, Andersen JT . The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J Control Release 2015; 211: 144–162.
Moriyama T, Takei T, Itabashi M, Uchida K, Tsuchiya K, Nitta K . Caveolae may enable albumin to enter human renal glomerular endothelial cells. J Cell Biol 2015; 116: 1060–1069.
Zloza A, Kim DW, Broucek J, Schenkel JM, Kaufman HL . High-dose IL-2 induces rapid albumin uptake by endothelial cells through Src-dependent caveolae-mediated endocytosis. J Interf Cytokine Res 2014; 34: 915–919.
Razzak M . Glomerular disease: albumin endocytosis is caveolin-mediated. Nat Rev Nephrol 2014; 10: 242.
Yumoto R, Nishikawa H, Okamoto M, Katayama H, Nagai J, Takano M . Clathrin-mediated endocytosis of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. Am J Physiol Lung Cell Mol Physiol 2006; 290: L946–L955.
Lambot N, Lybaert P, Boom A, Delogne-Desnoeck J, Vanbellinghen AM, Graff G et al. Evidence for a clathrin-mediated recycling of albumin in human term placenta. Biol Reprod 2006; 75: 90–97.
Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES . Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci USA 2009; 106: 2788–2793.
Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 2003; 197: 315–322.
Xiao X, Li J, Samulski RJ . Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998; 72: 2224–2232.
Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P . Non-classical anti-factor VIII C2 domain antibodies are pathogenic in a murine in vivo bleeding model. J Thromb Haemost 2009; 7: 658–664.
Acknowledgements
We are grateful to Xiaojing Chen and Karen Hogan for their excellent technical assistance. We acknowledge the UNC Biomedical Research Imaging Center (BRIC) Small Animal Imaging (SAI) facility for assistance of mouse imaging. This work was supported by National Institutes of Health Grants R01DK084033 and R01AI117408 (to CL and RJS), R01HL125749 (to CL), P01HL112761 and R01AI072176 (to RJS), P30-CA016086-35-37 and U54-CA151652-01-04 (to the BRIC SAI facility) and a research grant from Asklepios BioPharmaceutical (to CL). This research is based in part on work conducted using the UNC Michael Hooker Proteomics Center, which is supported in part by the NIH-NCI Grant Number CA016086 to the Lineberger Comprehensive Cancer Center.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R Jude Samulski is the founder and a shareholder at Asklepios BioPharmaceutical. He receives research support through the University of North Carolina from Asklepios BioPharmaceutical. He holds patents that have been licensed by UNC to Asklepios Biopharmaceutical, for which he receives royalties. He has consulted for Baxter Healthcare and has received payment for speaking. All other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, M., Sun, J., Crosby, A. et al. Direct interaction of human serum proteins with AAV virions to enhance AAV transduction: immediate impact on clinical applications. Gene Ther 24, 49–59 (2017). https://doi.org/10.1038/gt.2016.75
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.75
This article is cited by
-
Neutralisation of adeno-associated virus transduction by human vitreous humour
Gene Therapy (2021)
-
An engineered serum albumin-binding AAV9 capsid achieves improved liver transduction after intravenous delivery in mice
Gene Therapy (2020)
-
Engineering adeno-associated virus vectors for gene therapy
Nature Reviews Genetics (2020)
-
Adeno-associated virus-binding antibodies detected in cats living in the Northeastern United States lack neutralizing activity
Scientific Reports (2020)
-
Capsid-specific removal of circulating antibodies to adeno-associated virus vectors
Scientific Reports (2020)