Abstract
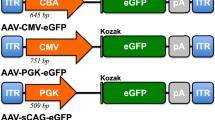
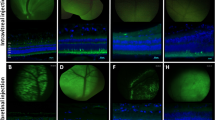
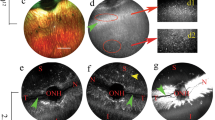
Delivery of therapeutic transgenes to retinal photoreceptors using adeno-associated virus (AAV) vectors has traditionally required subretinal injection. Recently, photoreceptor transduction efficiency following intravitreal injection (IVT) has improved in rodent models through use of capsid-mutant AAV vectors; but remains limited in large animal models. Thickness of the inner limiting membrane (ILM) in large animals is thought to impair retinal penetration by AAV. Our study compared two newly developed AAV vectors containing multiple capsid amino acid substitutions following IVT in dogs. The ability of two promoter constructs to restrict reporter transgene expression to photoreceptors was also evaluated. AAV vectors containing the interphotoreceptor-binding protein (IRBP) promoter drove expression exclusively in rod and cone photoreceptors, with transduction efficiencies of ~4% of cones and 2% of rods. Notably, in the central region containing the cone-rich visual streak, 15.6% of cones were transduced. Significant regional variation existed, with lower transduction efficiencies in the temporal regions of all eyes. This variation did not correlate with ILM thickness. Vectors carrying a cone-specific promoter failed to transduce a quantifiable percentage of cone photoreceptors. The newly developed AAV vectors containing the IRBP promoter were capable of producing photoreceptor-specific transgene expression following IVT in the dog.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nonnenmacher M, Weber T . Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther 2012; 19: 649–658.
Asokan A, Schaffer DV, Samulski RJ . The AAV vector toolkit: poised at the clinical crossroads. Mol Ther 2012; 20: 699–708.
Dalkara D, Sahel JA . Gene therapy for inherited retinal degenerations. C R Biol 2014; 337: 185–192.
Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 2012; 130: 9–24.
Nork TM, Murphy CJ, Kim CB, Ver Hoeve JN, Rasmussen CA, Miller PE et al. Functional and anatomic consequences of subretinal dosing in the cynomolgus macaque. Arch Ophthalmol 2012; 130: 65–75.
Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V et al. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis 2008; 14: 1760–1769.
Dudus L, Anand V, Acland GM, Chen SJ, Wilson JM, Fisher KJ et al. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision Res 1999; 39: 2545–2553.
Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci USA 2012; 109: 2132–2137.
Dalkara D, Byrne LC, Lee T, Hoffmann NV, Schaffer DV, Flannery JG . Enhanced gene delivery to the neonatal retina through systemic administration of tyrosine-mutated AAV9. Gene Ther 2012; 19: 176–181.
Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 2013; 5: 189ra76.
Kay CN, Ryals RC, Aslanidi GV, Min SH, Ruan Q, Sun J et al. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One 2013; 8: e62097.
Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology 2008; 381: 194–202.
Boye S, Bennett A, VanVliet K, Dinculescu A, White M, Peterson J et al. Heparan sulfate affinity dictates transduction of photoreceptors from the vitreous by capsid mutated AAV2 variants. Invest Ophthalmol Vis Sci 2014; 55: (abstract 3337).
Gabriel N, Hareendran S, Sen D, Gadkari RA, Sudha G, Selot R et al. Bioengineering of AAV2 capsid at specific serine, threonine, or lysine residues improves its transduction efficiency in vitro and in vivo. Hum Gene Ther Methods 2013; 24: 80–93.
Yin L, Greenberg K, Hunter JJ, Dalkara D, Kolstad KD, Masella BD et al. Intravitreal injection of AAV2 transduces macaque inner retina. Invest Ophthalmol Vis Sci 2011; 52: 2775–2783.
Mowat FM, Gornik KR, Dinculescu A, Boye SL, Hauswirth WW, Petersen-Jones SM et al. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther 2014; 21: 96–105.
Petersen-Jones SM, Komaromy AM . Dog models for blinding inherited retinal dystrophies. Hum Gene Ther Clin Dev 2015; 26: 15–26.
Beltran WA, Cideciyan AV, Guziewicz KE, Iwabe S, Swider M, Scott EM et al. Canine retina has a primate fovea-like bouquet of cone photoreceptors which is affected by inherited macular degenerations. PLoS One 2014; 9: e90390.
Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 2001; 28: 92–95.
Komaromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet 2010; 19: 2581–2593.
Annear MJ, Bartoe JT, Barker SE, Smith AJ, Curran PG, Bainbridge JW et al. Gene therapy in the second eye of RPE65-deficient dogs improves retinal function. Gene Ther 2011; 18: 53–61.
Mowat FM, Bartoe JT, Bruewer A, Dinculescu A, Boye SL, Hauswirth WW et al. Evaluation of rod photoreceptor function and preservation following retinal gene therapy in the PDE6A mutant dog. Invest Ophthalmol Vis Sci 2012; 53: (Abstract 1928).
Yeh C, Iwabe S, Boye S, McDaid K, Harman C, Wen R et al. Optimization of cone-directed AAV-mediated gene augmentation therapy for CNGB3-achromatopsia by use of the IRBP/GNAT2-promoter and intravitreal CNTF administration. Invest Ophthalmol Vis Sci 2013; 54: (Abstract 1937).
Ivanova E, Hwang GS, Pan ZH, Troilo D . Evaluation of AAV-mediated expression of Chop2-GFP in the marmoset retina. Invest Ophthalmol Vis Sci 2010; 51: 5288–5296.
Jayandharan GR, Zhong L, Li B, Kachniarz B, Srivastava A . Strategies for improving the transduction efficiency of single-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther 2008; 15: 1287–1293.
Byrne LC, Ozturk BE, Lee T, Fortuny C, Visel M, Dalkara D et al. Retinoschisin gene therapy in photoreceptors, Muller glia or all retinal cells in the Rs1h-/- mouse. Gene Ther 2014; 21: 585–592.
Schultz BR, Chamberlain JS . Recombinant adeno-associated virus transduction and integration. Mol Ther 2008; 16: 1189–1199.
Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 2007; 317: 477.
Tenenbaum L, Lehtonen E, Monahan PE . Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr Gene Ther 2003; 3: 545–565.
Dyka FM, Boye SL, Ryals RC, Chiodo VA, Boye SE, Hauswirth WW . Cone specific promoter for use in gene therapy of retinal degenerative diseases. Adv Exp Med Biol 2014; 801: 695–701.
Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, Schaffer DV et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther 2009; 17: 2096–2102.
Cehajic-Kapetanovic J, Le Goff MM, Allen A, Lucas RJ, Bishop PN . Glycosidic enzymes enhance retinal transduction following intravitreal delivery of AAV2. Mol Vis 2011; 17: 1771–1783.
Kolstad KD, Dalkara D, Guerin K, Visel M, Hoffmann N, Schaffer DV et al. Changes in adeno-associated virus-mediated gene delivery in retinal degeneration. Hum Gene Ther 2010; 21: 571–578.
Johnson TV, Bull ND, Martin KR . Transplantation prospects for the inner retina. Eye 2009; 23: 1980–1984.
Kim H, Robinson S, Csaky K . Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm Res 2009; 26: 329–337.
Koo H, Moon H, Han H, Na JH, Huh MS, Park JH et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials 2012; 33: 3485–3493.
Aartsen WM, van Cleef KW, Pellissier LP, Hoek RM, Vos RM, Blits B et al. GFAP-driven GFP expression in activated mouse Muller glial cells aligning retinal blood vessels following intravitreal injection of AAV2/6 vectors. PLoS One 2010; 5: e12387.
Matsumoto B, Blanks JC, Ryan SJ . Topographic variations in the rabbit and primate internal limiting membrane. Invest Ophthalmol Vis Sci 1984; 25: 71–82.
Sebag J . Anatomy and pathology of the vitreo-retinal interface. Eye 1992; 6: 541–552.
Wolter JR . Pores in the internal limiting membrane of the human retina. Acta Ophthalmol (Copenh) 1964; 42: 971–974.
Mutlu F, Leopold IH . The structure of human retinal vascular system. Arch Ophthalmol 1964; 71: 93–101.
Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ Jr et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 2002; 28: 158–167.
Ying S, Fong SL, Fong WB, Kao CW, Converse RL, Kao WW . A CAT reporter construct containing 277 bp GNAT2 promoter and 214 bp IRBP enhancer is specifically expressed by cone photoreceptor cells in transgenic mice. Curr Eye Res 1998; 17: 777–782.
Gearhart PM, Gearhart C, Thompson DA, Petersen-Jones SM . Improvement of visual performance with intravitreal administration of 9-cis-retinal in Rpe65-mutant dogs. Arch Ophthalmol 2010; 128: 1442–1448.
Mowat FM, Breuwer AR, Bartoe JT, Annear MJ, Zhang Z, Smith AJ et al. RPE65 gene therapy slows cone loss in Rpe65-deficient dogs. Gene Ther 2012; 20: 545–555.
Acknowledgements
We thank Janice Querbin and Kristen Koehl for animal assistance, Kristen Gervais and Laurence Occelli for imaging assistance and Tzu-Fen Chang and Joe Hauptman for statistical assistance. We also thank Cheryl Craft for hCAR antibody production, as well as Vince Chiodo and the Retinal Gene Therapy Vector lab for AAV purification. JTB and RFB acknowledge funding from Michigan State University College of Veterinary Medicine Endowed Research Fund. SLB, SEB, WWH, and AMK acknowledge funding from Foundation Fighting Blindness. SEB acknowledges funding from NIH Grant R01 EY024280. AMK acknowledges funding from NIH Grant R01 EY019304. SPJ acknowledges funding from Myers-Dunlap Endowment for Canine Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
WWH and the University of Florida have a financial interest in the use of AAV therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Boyd, R., Sledge, D., Boye, S. et al. Photoreceptor-targeted gene delivery using intravitreally administered AAV vectors in dogs. Gene Ther 23, 223–230 (2016). https://doi.org/10.1038/gt.2015.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.96
This article is cited by
-
Serotype-specific transduction of canine joint tissue explants and cultured monolayers by self-complementary adeno-associated viral vectors
Gene Therapy (2023)
-
Improving adeno-associated viral (AAV) vector-mediated transgene expression in retinal ganglion cells: comparison of five promoters
Gene Therapy (2023)
-
Retina transduction by rAAV2 after intravitreal injection: comparison between mouse and rat
Gene Therapy (2019)
-
Therapeutic perspectives for structural and functional abnormalities of cilia
Cellular and Molecular Life Sciences (2019)
-
Phenotypic characterization of complete CSNB in the inbred research beagle: how common is CSNB in research and companion dogs?
Documenta Ophthalmologica (2018)