Abstract
Development of curative approaches for HIV-1 infected patients requires novel approaches aimed at eliminating viral reservoirs and replacing potential target cells with infection-resistant immune cell populations. We have previously shown that autologous transplantation of genetically modified hematopoietic stem cells (HSCs) with lentiviral vectors encoding the mC46-fusion inhibitor results in a significant reduction in viral pathogenesis following challenge with the highly pathogenic dual tropic, SHIV89.6P strain. In this study, we used a combinatorial approach in which following engraftment of genetically modified HSCs, pigtailed macaques were vaccinated with a previously developed vaccinia-based vaccine expressing SIV-Gag, Pol. Using this dual therapy approach, lower viremia was detected in both the acute and chronic phase of disease with levels reaching near the lower limits of detection. In comparison with macaques receiving HSCT only, the combination approach resulted in a further log decrease in plasma viremia. Similar to our previous studies, positive selection of all CD4+ T-cell subsets was observed; however, higher gene-modified CD4+ T-cell levels were observed during the chronic phase when vaccination was included suggesting that combining vaccination with HSCT may lower the necessary threshold for achieving viremic control.
Similar content being viewed by others
Introduction
The development of curative therapies for HIV/AIDS requires novel approaches that eliminate viral reservoirs and replaces target cells with infection-resistant immune cell populations.1 Previous studies in HIV-1 infected patients have demonstrated that hematopoietic stem cell transplantation (HSCT) significantly reduced viral reservoirs; however, following the cessation of antiretroviral therapy, plasma viremia rebounds.2 In 2009, a study by Hutter et al.3 demonstrated that allogeneic transplantation from a CCR5null donor resulted in the first reported cure of an HIV-1 infected patient. Although this study has yielded promising results, allogeneic transplantation is associated with potentially severe adverse effects.4 Hence, we and others have developed methods to render hematopoietic stem cell (HSC)-derived immune cell populations resistant to HIV-infection (reviewed in Younan et al.5). We previously demonstrated that pigtailed macaques transplanted with genetically modified HSCs expressing the mC46-fusion inhibitor exhibited a significant reduction in viral pathogenesis.6, 7 In this study, our aim was to assess the potential for combining multiple approaches in an effort to increase the effectiveness over HSCT alone.
Several attenuated viral vectors have been utilized as delivery vehicles for viral antigens in both pre-clinical and clinical trials (reviewed in Goepfert et al.8 and Ondondo et al.9). The efficacy of many vaccines at producing a desirable immune response has been shown for multiple viral vectors designed to vaccinate against HIV-1. However, although promising results have been observed in animal models, to date an effective vaccine against HIV-1 has remained elusive. Findings from the previously conducted STEP trial indicated that although a measurable adaptive immune response was detectable in >80% of recipients, those that received adeno-based, Gag-pol vaccine exhibited a heightened susceptibility to HIV-1 infection (reviewed in Sekaly10). Though the mechanism for which remain unclear, it is feasible to predict that at least impart, HIV-1 specific CD4+ T-cells may contribute to the increased susceptibility as virus-specific CD4+ T-cells have previously been reported to be the initial target cells following exposure. Hence, in the case of HIV-1 infection, virus-specific CD4+ T-cells may fuel disease susceptibility.
Our previous studies provided initial evidence that mC46-expressing HSCs give rise to simian-human immunodeficiency virus (SHIV)-specific, infection-resistant CD4+ T-cells.7 These findings, which were based on ex vivo stimulation of peripheral blood mononuclear cell (PBMC) with SHIV-specific peptides, indicated that throughout the course of disease, infection-resistant CD4+ T-cells were maintained thereby providing their critical helper functions as was assessed by heightened and broadened cytotoxic T-lymphocytes and B-cell responses. These findings led us to speculate that vaccination following the engraftment of infection-resistant immune cell populations may increase the effectiveness of vaccinations following challenge with SHIV89.6P.
Results and Discussion
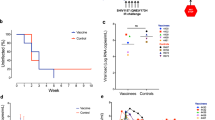
Recently, we showed that pigtailed macaques receiving autologous transplantation following genetic modification of HSCs presented significantly reduced viral pathogenesis following challenge with SHIV89.6P, a highly pathogenic dual tropic stain.7 In this study, we investigated the potential of a combinatorial approach in which previously transplanted pigtailed macaques were vaccinated with VV-SIVgag-pol following engraftment of genetically modified, mC46-expressing HSCs (Figure 1a). Successful vaccination was determined by enzyme-linked immunosorbent assay in all macaques following detection of SIV-specific antibodies (Supplementary Figure 4A). As indicated in Figure 1b, mC46-experimental pigtailed macaques receiving dual therapies exhibited a reduced peak plasma viremia at day 14 post challenge (4.9 × 106 v.RNA copies per ml) versus control (3.0 × 107 v.RNA copies per ml) during the acute phase of disease with viremia levels nearing the lower limit of detection of our assays during the chronic stages of disease (4.25 × 102 v.RNA copies per ml in mC46-experimental macaques versus 2.5 × 106 v.RNA copies/ml in a control macaque). As a comparison, mC46-experimental and control macaques receiving HSCT alone exhibited peak viremia levels of 1.9 × 108 and 2.3 × 108 v.RNA copies per ml, respectively, whereas plasma viremia levels were maintained at 4.4 × 103 and 1.5 × 106 v.RNA copies per ml during the chronic phase of infection (Figure 1b, weeks 8 through 24). Hence, a 50-fold decrease in peak plasma viremia was observed 2 weeks following challenge in dual therapy, mC46-macaques versus control and mC46-macaques receiving HSCT alone. A further 1 log reduction in plasma viremia was observed during the chronic phase of infection in dual therapy, mC46-macaques versus mC46-HSCT alone, whereas a remarkable 5884-fold decrease was observed in comparison with the control, dual therapy macaque. It was noted that a second control macaque receiving dual therapy exhibited a natural controller phenotype, and hence, this macaque was used for comparative purposes (Figure 1b, black diamonds). The natural controller exhibited peak and chronic plasma viremia levels of 5.6 × 105 and as low as 89 vRNA copies ml−1, respectively. Quantitative PCR analysis of DNA isolated from PBMC indicated that dual therapy, mC46-macaques maintained lower proviral DNA content then the natural controller throughout the course of infection (Supplementary Figure 1). Further confirming successful vaccination and a likely key contributing factor to the reduced plasma viremia levels was the rapid and pronounced increase in SHIV-specific antibody responses in dual therapy, mC46-macaques (Supplementary Figure 4B). Interestingly, although a similar shift in detectable antibodies was initially observed in the dual therapy control macaque, the magnitude and duration of the antibody response was greatly subdued in comparison with mC46-experimental macaques, suggesting that any benefits from pre-challenge exposure to viral antigens rapidly dissipated in the absence of mC46-expression.
Combination mC46-HSCT and vaccination reduces viral pathogenesis. (a) Diagram indicating study design. HSCT alone: macaques receive autologous HSCT following ex vivo genetic modification with mC46-encoding lentivirus or a control (both expressing GFP). HSCT+vaccination: macaques are vaccinated following identical HSCT procedures with VV-SIVgagpol at weeks 8 and 4 prior to challenge with SHIV89.6P. Analysis was performed over a 24-week period following SHIV-challenge. (b) Plasma viremia was assessed over the course of a 24-week period following SHIV89.6P challenge in control and experimental macaques following autologous HSCT. Control macaques (Cont HSCT) received infusion of autologous CD34+ HSCs modified with GFP-expressing lentiviral vectors (Cont HSCT) whereas a second control group (Cont-HSCT+VV-SIVGAGPOL) received two doses of a vaccinia-based vaccine following engraftment of gene-modified cells. Both experimental groups received HSCs transduced with lentiviral vectors encoding the mC46-fusion inhibitor in addition to GFP; the first group received HSCT alone (mC46-HSCT) whereas the second group was vaccinated (mC46-HSCT+VV-SIVGAGPOL). Note: one control, vaccinated macaque exhibited a natural controller phenotype (natural controller) and was therefore individually plotted. (c) The CD4+ T-cell count was assessed prior to and following SHIV89.6P challenge. (d) Ratio of CD4+ T-cells to CD8+ T-cells prior to and following SHIV89.6P challenge. Engraftment*—in vivo chemoselection was utilized to increase the percentage of genetically modified cells as needed.
As a consequence of decreased plasma viremia, peripheral CD4+ T-cells counts rapidly recovered following the acute phase of infection to near pre-infection levels in dual therapy mC46-macaques (Figure 1c). As observed previously in mC46-macaques receiving HSCT alone, CD4+ T-cell levels declined at a similar rate following SHIV-challenge in dual therapy macaques. On the basis of these findings, we suggest that neither HSCT alone nor dual therapy approaches can completely protect against the initial CD4+ T-cell depletion observed following infection as the majority of CD4+ T-cell depletion occurs as a consequence of bystander effects.11 However, when comparing both HSCT alone and dual therapy mC46-expressing macaques to control, HSCT alone macaques we noted a significantly reduced loss of CD4+ T-cells of ~1 log at week 3 post challenge (Figure 1c). In support of our hypothesis regarding the susceptibility of vaccinated macaques lacking infection-resistant immune cell population, the pigtailed macaque receiving control HSCT and VV-SIVgagpol exhibited the highest peak viremia levels during both the acute and chronic phase of infection. Peripheral CD4+ T-cell levels rapidly declined to near undetectable levels by week 3 in this macaque requiring early euthanasia due to the development of severe AIDS-like symptoms. CD4+ T-cells in both experimental macaques receiving combination therapy exhibited recovery kinetics similar to mC46-macaques receiving HSCT alone (Figure 1c). As a consequence of the recovery, a gradual increase in the ratio of CD4 to CD8 T-cells observed in dual therapy, mC46-expressing macaques (Figure 1d). A 3.4-fold decrease was observed 24 weeks post SHIV-challenge in dual mC46-expressing macaques versus a 3.8-fold decrease in HSCT alone, mC46-expressing macaques, suggesting that the rate of recovery in dual therapy macaques may be enhanced. Conversely, no increase in the CD4/CD8 ratio was observed in control macaques. Of note, the natural controller macaque maintained a relatively unchanged ratio of CD4 to CD8 T-cells (Figure 1d).
Analysis of the percentage of genetically modified CD4+ T-cells demonstrated that similar to HSCT alone mC46-macaques, mC46-expressing CD4+ T-cells in dual therapy mC46-macaques exhibited a definitive selective advantage following SHIV-challenge (Figure 2). Both mC46-expressing groups maintained >90% gene-modified CD4+ T-cells 3 weeks following SHIV89.6 P challenge (Figure 2a). Conversely, the levels of GFP-expressing CD4+ T-cells in control groups remained relatively unchanged over the course of these experiments; hence indicating a clear selective advantage of mC46-expression. Similar to our previous observations, the level of genetically modified CD4+ T-cells gradually decreased before plateauing at ~55% (Figure 2a). The mechanisms by which non-modified CD4+ T-cells recover remain unclear; however, it is important to note that selective advantage occurs in primary CD4+ T-cells and not at the HSC level. Hence, we suggest that following the initial depletion, the emergent non-modified CD4+ T-cells arising from non-modified HSCs do not encounter the same selective pressure as observed during the initial inflammatory burst that occurs during the acute phase of infection. In addition, the decreased plasma viremia observed in mC46-expressing macaques (Figure 1b) will undoubtedly contribute to the recovery of non-modified CD4+ T-cells. Interestingly, we noted that the 55% plateau was reached in both dual mC46/VV-SIVgagpol macaques that maintained ~20% gene-modified cells before SHIV-challenge. This represent an approximately twofold increase in the percentage of gene-modified cells observed during the plateau phase of a mC46-HSCT alone macaque that similarly maintained ~20% gene-modified cells before challenge and plateaued at ~30% during the chronic phase of infection. As assessed in Figure 3, the increase may be due to an overall increase in the percentage of self-renewing, memory CD4+ T-cells in addition to a continuous development of genetically modified, HSC-derived CD4+ T-cells.
Positive selection of genetically modified CD4+ T-cells. The percentage of gene-modified CD4+ T-cells was assessed at the indicated time points to determine the relative selective advantage of mC46-expression in (a) peripheral CD4+ T-cells and biopsies taken from (b) lymph nodes and (c) the GI tract.
Maintenance of CD4+ T-cell subsets in macaques receiving dual therapy. Individual CD4+ T-cell subpopulations were analyzed prior to and at weeks 3, 12 and 24 following SHIV89.6P challenge in the vaccinated groups of macaques. The percentage of (a) naive (b) central memory (c) effector memory and (d) transitional memory CD4+ T-cells was assessed by flow cytometry.
In addition to positive selection being observed in peripheral blood, positive selection of CD4+ T-cells was also observed in lymph nodes and gastrointestinal tract. Pre-challenge gene-modified levels of ~19% and 16% were observed in lymph nodes of mC46-alone and dual mC46/VV-SIVgagpol macaques, respectively (Figure 2b). Examination at 24 weeks post SHIV89.6P challenge revealed an increase of >40% in both groups, with ~60% genetically modified CD4+ T-cells being observed. Similarly, positive selection was observed in gastrointestinal samples taken from mC46-groups 24 weeks post SHIV89.6P challenge, with an average of ~60% and 52% being observed in HSCT alone and HSCT/VV-SIVgagpol groups (Figure 2c).
We previously demonstrated that mC46-expressing macaques maintained SHIV-specific, gene-modified CD4+ T-cell responders critical to the development of an adaptive immune response whereas control macaques were essentially devoid of these populations.7 Here, we examined the percentage of naive, central memory, effector memory and transitional memory CD4+ T-cells populations and determined the relative percentage of genetically modified cells within each subset in dual, HSCT/VV-SIVgagpol macaques. Examination of the naive population of CD4+ T-cells indicated that before SHIV89.6P challenge, the control macaque maintained ~29% gene-modified CD4+ T-cells, whereas both dual HSCT-vaccine macaques an average of ~21% and the macaque with the natural controller phenotype maintained ~21%. Three weeks following SHIV89.P challenge, naive CD4+ T-cells were undetectable in the control macaque, whereas the percentage naive CD4+ T-cells decreased to 15.1% in both mC46 dual therapy macaques (Figure 3a). The naive CD4+ T-cell subset increased in the natural controller macaque to 27.9%, which was unexpected as the burst in immunological responses would have been predicted to cause non-specific CD4+ T-cell activation; and hence, a decrease in the proportion of naive CD4+ T-cells. By week 12 however, the percentage of naive CD4+ T-cells increased to 20.4% in dual therapy, mC46-macaques and returned to near pre-infection levels 24 weeks post challenge with naive cells making up 18.4% of the total CD4+ T-cell population (Figure 3a). A decreasing trend in naive CD4+ T-cells was observed in the natural controller macaque with reduced levels of 16.5% being observed at week 24, indicating a gradual shift to other distinct subsets. The immune exhaustion marker PD1 and activation marker CD137 were elevated on CD4+ T-cells in dual therapy and the natural controller macaques 3 weeks following SHIV89.6P challenge; however, the upregulation of CD137 in dual therapy macaques was markedly higher, indicating an overall increase in CD4+ T-cell activation (Supplementary Figure 2). Both PD1 and CD137 expression returned to pre-infection levels by week 24 suggesting both groups of macaques had exhibited a controlled immune response.
Overall, the percentage of central memory CD4+ T-cells remained relatively unchanged in dual, HSCT/VV-SIVgagpol mC46-expressing macaques or the natural controller macaque at the time points examined (Figure 3b). As indicated in Figure 1c, the near-complete absence of CD4+ T-cells in the control, dual therapy macaque rendered the detection of various subsets undetectable following SHIV89.6P challenge. Interestingly, the decrease in naive CD4+ T-cells can be attributed to increases in both effector memory and transitional memory CD4+ T-cells (Figure 3c and d). TEM levels of 10.4, 10.7 and 10.1 were observed in dual therapy control, mC46-expressing and natural controller macaques before challenge (Figure 3c). TEM levels increased in dual, HSCT/VV-SIVgagpol mC46-macaques at week 12 (21.56%) before slightly decreasing at week 24 (20.6%). The development of this population of memory cells is critical for the maintenance of antigen-specific effector T-cell populations.12 The kinetics of expansion and contraction of this subset paralleled those detected in the natural controller macaque. The percentage of transitional memory CD4+ T-cells paralleled TEM development in dual therapy mC46-macaques (Figure 3d). After peaking at week 12 (13.5%), a slight decrease was observed at week 24 (13.2%). This finding suggests that effector memory CD4+ T-cells have shortened half-lives as compared with central memory cells. As indicated in Figure 1 and 3, mC46-expressing macaques are capable of maintaining CD4+ T-cells throughout the course of infection. To assess a possible mechanism of macaques in our dual therapy cohort to maintain high levels of memory CD4+ T-cells, we assessed the percentage of genetically modified cells within each memory subset. In parallel to the positive selection observed in Figure 2, we observed a substantial increase in the percentage of all memory subsets following SHIV89.6P challenge in dual therapy, mC46-macaques (Supplementary Figure 3). We suggest that increase in gene-modified, central memory CD4+ T-cells in particular is a critical finding as these cells undergo self-renewal.13 Although memory subsets were not directly assessed in our previous HSCT alone studies, we suggest that our dual vaccination approach may increase the percentage of infection-resistant TCM CD4+ T-cells following SHIV-challenge thereby accounting for the twofold increase in the percentage of gene-modified CD4+ T-cells during the chronic phase of infection. On the basis of our previous findings, we predict that the majority of SHIV-specific CD4+ T-cell responders are derived from these genetically modified, infection-resistant memory cell subsets.7
In conclusion, although a limited number of macaques were utilized in these preliminary studies, we show that combining HSCT-based therapies with previously developed vaccine strategies may greatly enhance the efficacy of HSCT-based monotherapy alone. The depletion of memory CD4+ T-cells has been associated with the progression to immunodeficiency leading to increased risk of opportunistic infections;12 hence, the maintenance of infection-resistant memory CD4+ T-cells may prevent the onset of immunodeficiency. Using this combination approach we were able to achieve a higher level of protection and reduced viral pathogenesis following SHIV89.6P challenge which, at least in part, may be attributable to the rapid and persistent production of virus-specific antibodies. Furthermore, we demonstrate that control pigtailed macaques receiving vaccination alone exhibit heightened susceptibility to SHIV-infection as both peak and viral set point during the acute and chronic stages of disease were elevated in comparison with infected naive macaques. These finding suggests that although HSC-based therapy alone and combination therapy may provide protection, vaccination alone could potentially lead to conditions that favor infection.
Materials and methods
Animals
Eight healthy juvenile pigtailed macaques (Macaca nemestrina) were housed at the University of Washington National Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Study protocols were approved by the Fred Hutchinson Cancer Research Center (FHCRC) institutional review board and the University of Washington Institutional Animal Care and Use Committee. Macaques were monitored closely and animal welfare was assessed on a daily basis and, if necessary, several times daily. If macaques experienced pain they received pain medications. Macaques were inoculated intravenously with a dose of 10 TCID50 SHIV89.6P.
HSCT and vaccination
Animal transplantation and ex vivo modification were conducted as previously described.14, 15 Briefly, animals were administered rhG-CSF at 100 μg kg−1, and also given recombinant human stem cell factor at 50 μg kg−1 prior to bone marrow collection. CD34+ cells were enriched by magnetic beads (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer's instructions with purity levels ranging from 85 to 99% CD34+ HSCs. A minimum of 107 CD34+ HSC kg−1 of body weight were transduced twice with lentiviral vectors at an MOI of 10 during a 48-h ex vivo culture period. All animals received myeloablative total-body irradiation, were infused with a minimum of 107 HSCs kg−1, and received rhG-CSF and standard supportive care following transplantation. Lentiviral constructs expressing mC46 (experimental only), MGMTP140K and GFP, ex vivo CD34+ transduction and in vivo selection of gene-modified HSC-derived lineages were previously described.6, 16 In all cases, macaques were allowed to recover and stabilize following chemotherapy administration (typically >3 months) prior to vaccination. Vaccination was performed as previously described.17 The recombinant vaccinia virus vaccine used in this study expresses SIV-gag and SIV-pol. Assessment of SHIV-specific antibody production was determined by enzyme-linked immunosorbent assay as previously described.17
Peripheral sampling, lymph node and gastrointestinal biopsies
Peripheral blood was collected at the indicated time points by venipuncture into heparin, EDTA or SST tubes for isolation of PBMCs and the isolation of plasma and serum samples. PBMCs were isolated from whole blood using a hemolytic lysis solution (ammonium chloride). Gut biopsies were obtained using an 8.9-mm diameter video gastroscope. A maximum of 23 pinch biopsies were taken per indicated time point using a 2.0-mm biopsy forceps. Single-cell suspensions were isolated following treatment of biopsies with 60 U ml−1 collagenase (Sigma Aldrich, St Louis, MO, USA). For axillary lymph node isolation, a small incision is made in the skin (<1 inch), the lymph node excised and the incision closed with non-absorbable suture. Cells are isolated following mechanical sheering of lymph nodes and lysis of red blood cells in hemolytic lysis buffer.
Plasma and cell-associated viral load assessment
Viral load was assayed as previously described. Briefly, viral RNA copy number was determined by real-time PCR following reverse transcription. Total viral DNA in PBMC was determined by real-time PCR and is expressed as the number of copies per 500 ng total DNA.
Lymphocyte immunophenotyping
Flow analysis was performed using the following antibodies: CD3-Ax700, CD4-PerCP-Cy5.5, CD8-APC-Cy7, CD137-APC, CCR5-PE, PD1, CD28, HLA-DR. All antibodies were purchased from BD Biosciences (San Jose, CA, USA). All samples were fixed with 1% paraformaldehyde prior to flow cytometric analysis on an LSR-II system (BD Biosciences).
References
Abou-El-Enein M, Bauer G, Reinke P, Renner M, Schneider CK . A roadmap toward clinical translation of genetically-modified stem cells for treatment of HIV. Trends Mol Med 2014; 20: 632–642.
Cillo AR, Krishnan A, Mitsuyasu RT, McMahon DK, Li S, Rossi JJ et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. J Acquir Immune Defic Syndr 2013; 63: 438–441.
Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360: 692–698.
Stan R, Zaia JA . Practical considerations in gene therapy for HIV cure. Curr HIV/AIDS Rep 2014; 11: 11–19.
Younan P, Kowalski J, Kiem HP . Genetically modified hematopoietic stem cell transplantation for HIV-1 infected patients: can we achieve a cure? Mol Ther 2014; 22: 257–264.
Trobridge GD, Wu RA, Beard BC, Chiu SY, Muñoz NM, von Laer D et al. Protection of stem cell-derived lymphocytes in a primate AIDS gene therapy model after in vivo selection. PLoS ONE 2009; 4: e7693.
Younan PM, Polacino P, Kowalski JP, Peterson CW, Maurice NJ, Williams NP et al. Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model. Blood 2013; 122: 179–187.
Goepfert P, Bansal A . Human immunodeficiency virus vaccines. Infect Dis Clin North Am 2014; 28: 615–631.
Ondondo BO . The influence of delivery vectors on HIV vaccine efficacy. Front Microbiol 2014; 5: 439.
Sekaly RP . The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med 2008; 205: 7–12.
Matrajt L, Younan PM, Kiem HP, Schiffer JT . The majority of CD4+ T-cell depletion during acute simian-human immunodeficiency virus SHIV89.6P infection occurs in uninfected cells. J Virol 2014; 88: 3202–3212.
Okoye AA, Picker LJ . CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev 2013; 254: 54–64.
Youngblood B, Hale JS, Ahmed R . T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology 2013; 139: 277–284.
Kiem H-P, Heyward S, Winkler A, Potter J, Allen JM, Miller AD et al. Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudotyped retroviral vectors in a competitive repopulation assay in baboons. Blood 1997; 90: 4638–4645.
Trobridge G, Beard BC, Kiem H-P . Hematopoietic stem cell transduction and amplification in large animal models. Hum Gene Ther 2005; 16: 1355–1366.
Kiem H-P, Wu RA, Sun G, von Laer D, Rossi JJ, Trobridge GD . Foamy combinatorial anti-HIV vectors with MGMTP140K potently inhibit HIV-1 and SHIV replication and mediate selection in vivo. Gene Ther 2010; 17: 37–49.
Hu SL, Zarling JM, Chinn J, Travis BM, Moran PA, Sias J et al. Protection of macaques against simian AIDS by immunization with a recombinant vaccinia virus expressing the envelope glycoproteins of simian type D retrovirus. Proc Natl Acad Sci USA 1989; 86: 7213–7217.
Acknowledgements
We are grateful for research funding from the National Institutes of Health, Bethesda, MD, grants R01 AI080326, U19 AI096111, R01 HL098489, P30 DK056465, AI027757 and P51 RR00016. We also acknowledge Veronica Nelson, Erica Wilson, Kelvin Sze and Heather Mack for their contributions to this study.
Author contributions
H-PK is the principal investigator of the study and together with PMY conceived and designed these studies. PMY and PP coordinated the macaque studies. PMY and JPK contributed substantially to data collection and analysis. S-LH and H-PK contributed substantially to the study conception, design and critically reviewed this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Younan, P., Polacino, P., Kowalski, J. et al. Combinatorial hematopoietic stem cell transplantation and vaccination reduces viral pathogenesis following SHIV89.6P-challenge. Gene Ther 22, 1007–1012 (2015). https://doi.org/10.1038/gt.2015.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.83