Abstract
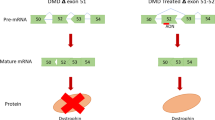
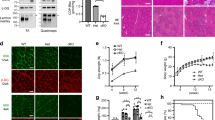
Antisense therapy with both chemistries of phosphorodiamidate morpholino oligomers (PMOs) and 2′-O-methyl phosphorothioate has demonstrated the capability to induce dystrophin expression in Duchenne muscular dystrophy (DMD) patients in phase II-III clinical trials with benefit in muscle functions. However, potential of the therapy for DMD at different stages of the disease progression is not understood. In this study, we examined the effect of peptide-conjugated PMO (PPMO)-mediated exon skipping on disease progression of utrophin-dystrophin-deficient mice (dko) of four age groups (21–29, 30–39, 40–49 and 50+ days), representing diseases from early stage to advanced stage with severe kyphosis. Biweekly intravenous (i.v.) administration of the PPMO restored the dystrophin expression in nearly 100% skeletal muscle fibers in all age groups. This was associated with the restoration of dystrophin-associated proteins including functional glycosylated dystroglycan and neuronal nitric synthase. However, therapeutic outcomes clearly depended on severity of the disease at the time the treatment started. The PPMO treatment alleviated the disease pathology and significantly prolonged the life span of the mice receiving treatment at younger age with mild phenotype. However, restoration of high levels of dystrophin expression failed to prevent disease progression to the mice receiving treatment when disease was already at advanced stage. The results could be critical for design of clinical trials with antisense therapy to DMD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoffman EP, Brown RH Jr, Kunkel LM . Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51: 919–928.
Konieczny P, Swiderski K, Chamberlain JS . Gene and cell-mediated therapies for muscular dystrophy. Muscle Nerve 2013; 47: 649–663.
Sherratt TG, Vulliamy T, Dubowitz V, Sewry CA, Strong PN . Exon skipping and translation in patients with frameshift deletions in the dystrophin gene. Am J Hum Genet 1993; 53: 1007–1015.
Dunckley MG, Manoharan M, Villiet P, Eperon IC, Dickson G . Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum Mol Genet 1998; 7: 1083–1090.
Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Fletcher S et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci USA 2001; 98: 42–47.
Dickson G, Hill V, Graham IR . Screening for antisense modulation of dystrophin pre-mRNA splicing. Neuromuscul Disord 2002; 12 (Suppl 1): S67–S70.
Aartsma-Rus A, Bremmer-Bout M, Janson AA, den Dunnen JT, van Ommen GJ, van Deutekom JC . Targeted exon skipping as a potential gene correction therapy for Duchenne muscular dystrophy. Neuromuscul Disord 2002; 12 (Suppl 1): S71–S77.
Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med 2003; 9: 1009–1014.
Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA 2005; 102: 198–203.
Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med 2006; 12: 175–177.
Abes R, Arzumanov AA, Moulton HM, Abes S, Ivanova GD, Iversen PL et al. Cell-penetrating-peptide-based delivery of oligonucleotides: an overview. Biochem Soc Trans 2007; 35: 775–779.
Amantana A, Moulton HM, Cate ML, Reddy MT, Whitehead T, Hassinger JN et al. Pharmacokinetics, biodistribution, stability and toxicity of a cell-penetrating peptide-morpholino oligomer conjugate. Bioconjug Chem 2007; 18: 1325–1331.
Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Spurney CF et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA 2008; 105: 14814–14819.
Jearawiriyapaisarn N, Moulton HM, Buckley B, Roberts J, Sazani P, Fucharoen S et al. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol Ther 2008; 16: 1624–1629.
Yin H, Moulton HM, Seow Y, Boyd C, Boutilier J, Iverson P et al. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet 2008; 17: 3909–3918.
Wu B, Li Y, Morcos PA, Doran TJ, Lu P, Lu QL . Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol Ther 2009; 17: 864–871.
Aoki Y, Nakamura A, Yokota T, Saito T, Okazawa H, Nagata T et al. In-frame dystrophin following exon 51-skipping improves muscle pathology and function in the exon 52–deficient mdx mouse. Mol Ther 2010; 18: 1995–2005.
Wu B, Lu P, Benrashid E, Malik S, Ashar J, Doran TJ et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Therapy 2010; 17: 132–140.
Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol 2009; 65: 667–676.
Moulton HM, Moulton JD . Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim Biophys Acta 2010; 1798: 2296–2303.
Aoki Y, Yokota T, Nagata T, Nakamura A, Tanihata J, Saito T et al. Bodywide skipping of exons 45–55 in dystrophic mdx52 mice by systemic antisense delivery. Proc Natl Acad Sci USA 2012; 109: 13763–13768.
Wu B, Xiao B, Cloer C, Shaban M, Sali A, Lu P et al. One-year treatment of morpholino antisense oligomer improves skeletal and cardiac muscle functions in dystrophic mdx mice. Mol Ther 2011; 19: 576–583.
Wu B, Lu P, Cloer C, Shaban M, Grewal S, Milazi S et al. Long-term rescue of dystrophin expression and improvement in muscle pathology and function in dystrophic mdx mice by peptide-conjugated morpholino. Am J Pathol 2012; 181: 392–400.
van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med 2007; 357: 2677–2686.
Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol 2009; 8: 918–928.
Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med 2011; 364: 1513–1522.
Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, Anthony K et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 2011; 378: 595–605.
Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Annals of Neurology 2013; 74: 637–647.
Bulfield G, Siller WG, Wight PA, Moore KJ . X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 1984; 81: 1189–1192.
Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 1997; 90: 717–727.
Porter JD, Rafael JA, Ragusa RJ, Brueckner JK, Trickett JI, Davies KE . The sparing of extraocular muscle in dystrophinopathy is lost in mice lacking utrophin and dystrophin. J Cell Sci 1998; 111: 1801–1811.
Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med 2006; 12: 787–789.
Marshall JL, Holmberg J, Chou E, Ocampo AC, Oh J, Lee J et al. Sarcospan-dependent Akt activation is required for utrophin expression and muscle regeneration. J Cell Bio 2012; 197: 1009–1027.
Laws N, Hoey A . Progression of kyphosis in mdx mice. J Appl Physiol 2004; 97: 1970–1977.
Acknowledgements
This work was supported by the Carolinas Muscular Dystrophy Research Endowment at the Carolinas HealthCare Foundation and Carolinas Medical Center, Charlotte, NC, Foundation to Eradicate Duchenne, Department of Defense (W81XWH-09-1-0599), and NIH/NICHD (U54 HD 071601-02). We thank AVI Biopharma (now Sarepta Therapeutics) for the supply of PPMOE23 for this study and Dr Mark Grady (Washington University, St Louis) and Dr Joshua Sanes (Harvard University, Cambridge) for providing mdx female mice heterozygous for utrophin (mdx;utr+/−).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Wu, B., Cloer, C., Lu, P. et al. Exon skipping restores dystrophin expression, but fails to prevent disease progression in later stage dystrophic dko mice. Gene Ther 21, 785–793 (2014). https://doi.org/10.1038/gt.2014.53
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.53
This article is cited by
-
Photobiomodulation therapy protects skeletal muscle and improves muscular function of mdx mice in a dose-dependent manner through modulation of dystrophin
Lasers in Medical Science (2018)
-
Combination Antisense Treatment for Destructive Exon Skipping of Myostatin and Open Reading Frame Rescue of Dystrophin in Neonatal mdx Mice
Molecular Therapy (2015)
-
Forelimb Treatment in a Large Cohort of Dystrophic Dogs Supports Delivery of a Recombinant AAV for Exon Skipping in Duchenne Patients
Molecular Therapy (2014)