Abstract
Oncolytic vaccinia virus is an attractive platform for immunotherapy. Oncolysis releases tumor antigens and provides co-stimulatory danger signals. However, arming the virus can improve efficacy further. CD40 ligand (CD40L, CD154) can induce apoptosis of tumor cells and it also triggers several immune mechanisms. One of these is a T-helper type 1 (Th1) response that leads to activation of cytotoxic T-cells and reduction of immune suppression. Therefore, we constructed an oncolytic vaccinia virus expressing hCD40L (vvdd-hCD40L-tdTomato), which in addition features a cDNA expressing the tdTomato fluorochrome for detection of virus, potentially important for biosafety evaluation. We show effective expression of functional CD40L both in vitro and in vivo. In a xenograft model of bladder carcinoma sensitive to CD40L treatment, we show that growth of tumors was significantly inhibited by the oncolysis and apoptosis following both intravenous and intratumoral administration. In a CD40-negative model, CD40L expression did not add potency to vaccinia oncolysis. Tumors treated with vvdd-mCD40L-tdtomato showed enhanced efficacy in a syngenic mouse model and induced recruitment of antigen-presenting cells and lymphocytes at the tumor site. In summary, oncolytic vaccinia virus coding for CD40L mediates multiple antitumor effects including oncolysis, apoptosis and induction of Th1 type T-cell responses.
Similar content being viewed by others
Introduction
Immunotherapy of cancer has resulted in recent clinical successes validating the potential of the approach. A key realization has been that in addition to induction of an antitumor immune response, reduction of tumor immune suppressiveness is also required. Oncolytic vaccinia virus seems a promising platform for immunotherapy. However, given its expression of anti-inflammatory molecules,1, 2, 3 an ‘arming’ strategy with immunostimulatory molecules is useful to maximize the immunotherapeutic effect.
Vaccinia virus (vv) is a genetically complex DNA virus encoding a large number of genes, some of which have immune-evading properties allowing the virus to establish local pockets of infection within an infected host. In the current work, we have used a Western Reserve double-deleted vaccinia virus (vvdd) that is restricted to tumor cell growth as it has deletions in the virally encoded thymidine kinase and vaccinia growth factor (VGF) genes.4 These mutations restrict virus replication to cells that overexpress E2F (the transcription factor that regulates cellular TK expression) and have activated epithelial growth factor receptor pathways.5
Antigen-presenting cells (APCs) such as dendritic cells (DCs) present antigens to T cells and have the ability to determine between immune response and tolerance. Normally, peptides derived from endogenously expressed proteins are presented by APC in the context of MHC class I (MHC I) to CD8+ T cells, whereas peptides obtained from exogenously derived proteins are normally loaded onto MHC class II (MHC II) for presentation to CD4+ T cells. However, exogenous antigens can be also loaded onto MHC I for ‘cross-presentation’ to CD8+ T cells.6
In tumor-draining lymph nodes, both cross-priming and cross-tolerization have been reported, tumor antigen-specific T-cell proliferation has been detected, but the numbers of T cells proliferating are often too low, and therefore the overall effect of CD8+ T-cell activation does not always result in inhibition of tumor growth.7
High expression of co-stimulatory factors that act directly on T cells has been proposed to enhance T-cell activation. CD154, also known as CD40L, is one such molecule. Normally it binds to CD40 on APC, which can trigger various signaling cascades on the target cell. In general, CD40L functions as a co-stimulatory molecule and induces activation in APC in association with T-cell receptor stimulation by MHC molecules.8 In addition to its effect on the immune system, CD40L also promotes direct apoptosis of CD40+ cells.9, 10, 11 Recombinant CD40L has been used in trials, with some efficacy, but systemic adverse events limited the dose that could be achieved locally, resulting in suboptimal efficacy.12 Monoclonal antibodies against CD40 have also provided exciting proof-of-concept data.12
Although CD40L as arming device has been explored in the context of other viral platforms such as adenoviruses13, 14, 15, 16, 17 or other gene therapy approaches,18, 19, 20 the combination of the oncolytic efficacy of vvdd and the immunological effects of CD40L have not been fully studied.
Recent observations have also underlined the importance of the type of death tumor cells undergo. The immunogenicity of cell death can significantly influence subsequent antitumor immune response and the overall efficacy of a drug.21, 22, 23 Specifically, it has been suggested that the translocation of the endoplasmic reticulum resident calreticulin–ERp57 complex to the plasma membrane is useful for immunogenic cell death.24 Subsequently, it was shown that the nuclear alarmin HMGB1 has to be released into the tumor microenvironment to engage TLR4 on host DCs to facilitate antigen processing and presentation.25 Finally, it was reported that ATP release from dying cells stimulates DCs for T-cell priming.26 This emerging theory applies also to biological drugs including oncolytic viruses.17
Results
Generation of human and murine CD40L-expressing vaccinia virus
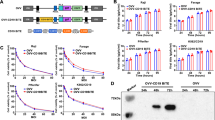
CD40 ligand (CD40L, CD154) can induce apoptosis of tumor cells and it also able to trigger several immune mechanisms. One of these is a T-helper type 1 (Th1) response that leads to activation of cytotoxic T-cells and reduction of immune suppression. The advantages of CD40L-expressing oncolytic viruses16, 17 together with encouraging results with oncolytic vaccinia virus27, 28, 29 lead us to generate novel oncolytic vaccinia virus expressing hCD40L (vvdd-tdtomato-hCD40L). To gain a more in-depth understanding of the mechanisms of CD40L in modulating the immune response, we generated also the same virus encoding murine CD40L (vvdd-tdtomato-mCD40L). Viruses are based on Western Reserve vaccinia viruses featuring deletions in the thymidine kinase (TK) and vaccinia growth factor (VGF) genes for improved cancer cell selective replication. (Transgenes were driven by vaccinia virus P7.5 or the synthetic PE/L promoters. In contrast to previous Western Reserve strain-based vaccinia designs, which have only featured an insertion into TK, we engineered a deletion of the TK region to completely avoid the possibility of back-recombination, which could result in a wild-type TK gene (Figure 1a). The virus genome also includes a cDNA expressing the tdTomato fluorochrome in order to facilitate identification of the virus in vitro using fluorescence microscopy (Figure 1b) and in vivo from subcutaneous tumors using imaging devices30 (Figure 1c). Compared with prior commonly used flurophores, such as green fluorescent protein, tdTomato possesses greater tissue penetration.31
Virus construct. (a) Schematic drawing of virus construct vvdd-tdtomato and vvdd-CD40L-tdtomato. Unlike in previous designs (where a mere insertion was employed), a deletion was engineered into the TK open reading (star). (b) In vitro expression of tdTomato 48 h after infection. (c) In vivo expression of tdTomato 48 h. Virus was intratumorally injected in nude mice bearing M4A4-LM3 breast tumors and imaged with a specially constructed in vivo fluorescent imaging device.
The expression of the the CD40L transgene does not compromise the oncolytic activity of the viruses
As it has been shown that CD40L can have antiviral activity,32 we assessed whether the oncolytic activity of the newly generated virus had remained unaltered. Different cell lines were infected with vaccinia vvdd-hCD40L-tdtomato and vvdd-tdtomato and assayed cell viability (Figure 2). Complete cell killing of A549, M4A4-lm3 and EJ cells was seen after 3 days at a dose of 1 p.f.u. per cell. Interestingly, we did not observe any significant difference between the two viruses in any of the tested cell line, indicating that the expression of this transgene did not significantly altered the biology of the virus that was still able to infect and kill human cancer cell line. When oncolytic effect of vvdd-mCD40L-tdtomato was assessed in comparison with the unarmed virus, viruses were equally oncolytic and B16-ova cells were completely killed 3 days after infection at a dose of 1 p.f.u. per cell (Supplementary Figure 2), indicating that also vvdd-mCD40L-tdtomato retains oncolytic potency in vitro.
The expression of the hCD40L transgene does not abrogate oncolytic efficacy. (a) Human lung adenocarcinoma cells (A549, CD40-), (b) bladder cancer cells (EJ, CD40+) and (c) breast cancer cells (M4A4-LM3, CD40-) were infected with different concentrations of replication-competent vvdd-tdtomato or vvdd-hCD40L-tdtomato. Three days later, cell viability was measured using an MTS assay.
vvdd-hCD40L-tdTomato promotes a more immunogenic form of cell death compared with control virus
Calreticulin exposure as well as ATP and HMGB1 release have been recently proposed as in vitro measurable indication of an immunogenic cell death.33 We have previously shown that an oncolytic adenovirus-expressing hCD40L is able to stimulate immunogenic cell death as measured through the release of these markers.17 HMGB and ATP release to the supernatant as well as calreticulin exposure on the cell surface was analyzed following 12 h after infection with vvdd-hCD40L-tdTomato or vvdd-tdtomato. We found that CD40+ EJ cells were responsive to vvdd-hCD40L-tdTomato, although only the enhancement of calreticuline exposure on EJ cells was considered statistically significant (Figures 3a–c). We tried to repeat the analysis also with later time points, but results were confused by the fact that oncolysis mediated by the virus starts killing the cells, masking the effect of CD40L, which was the purpose of these sets of experiments. However, it is possible that the release of HMGB1 and ATP should ideally be analyzed at later time points.34
vvdd-hCD40L-tdTomato displays tumor-restricted expression of the transgene
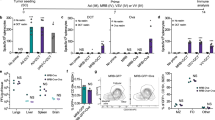
One of the most important advantages of using armed oncolytic viruses is their capability to replicate and express the transgene locally at the tumor site for increased local efficacy while reducing side effects due to high systemic concentrations. We performed several in vitro and in vivo experiments to study the expression of hCD40L from vvdd-hCD40L-tdTomato. A549 cell line was infected with different concentrations of the virus, and human CD40L was measured in the media at different time points (Figure 4a). vvdd-tdtomato was used as control to exclude that A549 could produce CD40L after infection per se. A dose-dependent increase of hCD40L was observed in infected cells (Figure 4a). Next, we sought to study the pharmacokinetics of transgene expression in vivo in tumor-bearing mice. To this end, nude mice were implanted with M4A4-LM3 breast tumors that were then injected with vvdd-hCD40L-tdTomato or control. At different time points, expression of tomato was analyzed by IVIS machine and simultaneously mice were bled for hCD40L quantification (Figures 4b, c and Supplementary Figure S1).
In vitro and in vivo expression of hCD40L. (a) A549 cells were infected with different p.f.u. per cell of vvdd-hCD40L. Supernatant was collected at different time points and analyzed by FACSarray for expression of hCD40L. Nude mice bearing M4A4-LM3 breast tumors were intratumorally injected with vvdd-tdTomato or vvdd-hCD40L-tdTomato-tdTomato. (b) Mice were imaged by IVIS. (c) Blood samples were collected on days 3 and 13, and tumors were collected on day 13. Blood and tumors were analyzed for hCD40l concentration with FACSarray.
As expected, no difference in tdtomato expression was observed between the group of mice treated with vvdd-tdTomato and vvdd-hCD40L-tdTomato, confirming comparable transgene expression (Figure 4b). Simultaneously, ELISA for hCD40L was performed to quantify the protein in the serum and in the tumor. Interestingly, although hCD40L was undetectable in the serum at any assessed time points, high levels of hCD40L were found in the tumor at day 13 (Figure 4c). These results show that the expression of the transgene is tumor restricted, and they highlight the advantage of using oncolytic viruses as a delivery system when tumor-restricted expression is desired.
vvdd-hCD40L-tdTomato results in increased antitumor activity in CD40-positive tumors following intratumoral and intravenous administration
We next sought to investigate the antitumor activity of vvdd-hCD40L-tdTomato in vivo. Nude mice bearing bladder cancer xenografts (EJ cell line, CD40 positive) were injected intratumorally. The CD40L-expressing virus showed significantly (P=0.031) increased antitumor activity compared with the unarmed virus, and all tumors were eventually cured (Figure 5a). In addition, as both viruses express tdTomato, virus replication could be followed by IVIS (Supplementary Figure S1a). Interestingly, when the experiment was repeated in a CD40-negative cell line, vvdd-hCD40L-tdTomato lost its advantage over its paternal unarmed virus (Figure 5b).
vvdd-hCD40L-tdtomato showed increased antitumor efficacy in CD40-positive tumors following intratumoral administration. Nude mice bearing human tumors were treated with PBS, vvdd-tdTomato and vvdd-hCD40L-tdtomato, and tumor growth was measured over time. Administration of the virus was performed intratumorally. (a) hCD40L-sensitive breast tumors (EJ cell line). (b) hCD40 negative tumors (A549 cell line).
We next wanted to assess the efficacy of the armed virus in the more challenging condition of intravenous administration. To this end, an experiment similar to the one described in Figure 5 was performed, but viruses were intravenously administered. Tumor growth in CD40-sensitive (Figure 6a) and non-sensitive (Figure 6b) were assessed. In addition, virus targeting and replication in the tumor were also measured (Supplementary Figure S1b). Although both viruses seemed to be efficacious concerning their antitumor effect, no difference was observed between the two viruses following intravenous administration. There are many obstacles to successful systemic delivery of viruses. For example, blood cells, complement and antiviral cytokines, as well as nonspecific uptake by other tissues such as the lung, liver, spleen and macrophages, might prohibit the systemic delivery of the virus.
Efficacy of vvdd-hCD40L-tdTomato in CD40-negative tumors following intravenous administration. Nude mice bearing human tumors were treated with PBS, vvdd-tdTomato and vvdd-hCD40L-tdTomato, and tumor growth was measured over time. Administration of the virus was performed intravenously. (a) hCD40L-sensitive breast tumors (EJ cell line). (b) hCD40 negative tumors (A549 cell line).
vvdd-hCD40L-tdTomato activates human lymphocytes and boosts their cytokine production
In order to investigate the effect of vvdd-hCD40L-tdTomato on the human immunological cells, we performed a set of experiments using human lymphocytes. In the first experiment, we used human a B-cell derived cell line (Burkitt’s lymphoma) stably expressing an NF-κB/AP-1-inducible secreted embryonic alkaline phosphate reporter construct (Ramos-Blue cells). This cell line is responsive to human CD40L and expresses alkaline phosphatase when activated. We cultured these cells with media filtered from A549 oncolysates (cells infected with oncolytic viruses). We found significantly enhanced activation when media derived from vvdd-hCD40L-tdTomato oncolysate was used (Figure 7a).
vvdd-hCD40L-tdTomato activates human lymphocytes and boosts their cytokine production. (a) Human-derived lymphocytes are activated by the vaccinia virus expressed hCD40L. Filtered supernatant from A549 cells infected with different concentrations of vvdd-tdTomato or vvdd-hCD40L-tdTomato was used to stimulate Ramos-blue cells. After 48 h, NFkB activation was determined using QUANTI-Blue. (b) Human PBMCs were stimulated with oncolysate from A549-infected cell line. Medium was collected, and cytokines were assessed by FACSARRAY.
In order to translate these findings in more clinically relevant experiment, we repeated the experiment with human primary lymphocytes and followed their immunogenic cytokine profile over time. Interestingly, PBMCs treated with lysate from vvdd-hCD40L-tdTomato showed higher secretion of RANTES at 24-h time point, which is an indicator of T-helper cell type immunity and suggest induction of a cytotoxic T-cell response. (Figure 7b).
Antitumor activity of CD40L in a syngenic immunocompetent mouse model
In immunocompetent mice with subcutaneous B16-ova murine melanoma tumors, a significant increase in antitumor activity was seen in the groups treated with vvdd-tdtomato and vvdd-mCD40L-tdtomato (Figure 8). Mice treated with vvdd-mCD40L-tdtomato had also significantly increased overall survival (P=0.0191) compared with mock until day 60 when the follow-up was terminated (Supplementary Figure S3). However, the difference between the groups receiving virus treatment or the difference between mock and vvdd-tdTomato was not considered statistically different. An important part of antitumor activity of CD40L is its effect on antigen-presenting cells and T cells. Flow cytometry analysis of the tumors revealed that mCD40L induces antitumor immune responses by recruiting lymphocytes, B-cells, dendritic cells and macrophages at the tumor site (Figure 9). Although only the induction of NK-cells and dendritic cells were statistically significant, these findings may indicate that production of mCD40L in syngeneic B16-ova tumors prompted a strong antitumor immune response mediated through Th1 responsive elements and cytotoxic T-cell infiltration. B cells were also significantly increased in the tumor. CD40-CD40L interactions are known to have a role in potentiating B cells accompanied by B-cell proliferation and differentiation for consequent induction of humoral responses. Activation of negative regulators of antitumor T-cell responses was also assessed by analyzing the amount of myeloid-derived suppressor cells (MDSC) in tumors. Athough MDSCs were upregulated in tumors, they did not seem to hamper the overall efficacy of the treatment. Stonger immune response caused by the CD40L-coding viruses resulted in a stronger suppressive signal in compensation, as is typical for the immune system.
Effects of CD40L in the tumors of B16-ova-bearing mice. Tumors were collected 18 days after first virus injection, smashed and cultured for 24 h. Analysis of cell markers was performed by flow cytometry (a) CD3, (b) CD4, (c) CD8, (d) NK-cells, (e) dendritic cells and (f) macrophages, and (g) B-cells and (h) myeloid-derived suppressor cells.
Spleens, tumor-draining lymph nodes and distal lymph nodes from the other flank side were also analyzed for T-cells, B-cells, macrophages and dendritic cells. Few B cells compared with mock were seen in tumor-draining lymph nodes and spleens (Supplementary Figures S4g and S5g). In tumor-draining lymph nodes, the number of CD8+ cells and macrophages was increased (Supplementary Figures S5c and f), which was not seen in distal draining lymphnode (Supplementary Figures S6c and f).
Discussion
In the present study, we report the results of the first human CD40 ligand expressing Western Reserve strain double-deleted vaccinia virus. The virus that we have generated presents several attractive features compared with a similar virus that has been used in several clinical trials (JX-594).29 First, our virus bears a double deletion, thymidine kinase (TK) and vaccinia growth factor (VGF). In addition, we have disrupted the TK gene not only by insertion of a cDNA as in earlier constructs but also by engineering, performing a partial deletion of DNA. This completely avoids any possibility of reconstitution of the wild-type TK gene in the event of expulsion of the transgene, which has been reported to occur. Although it has never been formally reported, we can speculate that ‘evolutionally’ the virus without the transgene that disrupt TK gene would have an advantage over the recombinant virus. In fact, we have noticed that the purification of vvdd starting with our shuttle plasmids requires less passage, and it is significantly more rapid than then purification of vvdd based on the other shuttle plasmid where TK is only disrupted but not deleted.4
Another interesting new characteristic of our new virus is the use of tdTomato for virus identification. tdTomato bears excellent features such an increased photostability and better penetrance in the tissue.35 This allows a better visualization of the virus in vitro and in vivo.30, 31
In a recent trial featuring another vaccinia strain, GFP was utilized to demonstrate that pox-lesions in the skin and mucous membranes of patients had virus and were not just reactive (Harrington K et al., oral communication at Oncolytic Virus Meeting, Las Svegas NV 2011). Without the fluorophore, the biosafety implications of the lesions might have been missed. Therefore, to understand the safety and shedding of oncolytic viruses, it could be quite important to have a non-invasively imageable transgene incorporated. The superior tissue penetration of tdTomato could be useful in case of lesions or adverse events occurring deeper than the skin. The imaging device we developed for imaging mice can be used in a human trial featuring a tdTomato-coding virus to evaluate presence, persistence and amplification in tumor versus normal tissues. While formal toxicity studies are needed, tdTomato is not expected to be toxic.30, 31, 35
Vaccinia virus has legendary immunogenicity due to its use in eradication of small pox. However, this was achieved mainly through antibody induction, and in fact vaccinia per se is not very potent in inducing cellular immunity.1, 36 It has a complex genome that encodes for several immunomodulatory proteins, including B18R, which naturally antagonizes innate cellular and antiviral responses initiated by type I interferons.1 Its immunomodulatory properties make this virus an intriguing platform to express immune stimulatory molecules by rationale design.
To boost antitumor immune responses, we armed vaccinia virus with the human CD40 ligand (hCD40L). As hCD40L is not active in mice, a virus encoding murine CD40 ligand (mCD40L) was generated in order to study the immunological aspects of the approach. We hypothesized that enhancing the co-stimulatory molecules on CD40-expressing cells such as APCs (mainly macrophages and dendritic cells) would increase cross-priming of T cells. To our knowledge, this is the first report on oncolytic vaccinia virus-expressing CD40L. The only similar approach was taken by Ruby et al. where they have studied the antiviral activity of CD40L cloning such molecule in a non oncolytic replication-competent vaccinia virus.32 They demonstrate that IL4-CD40L-expressing VV was attenuated to the extent that even immunocompromised mice could survive lethal infections.32 These results are intriguing and simultaneously encouraging since they demonstrate that the CD40L-expressing vvdd virus might have even an additional extra-safety compared with unarmed vvdd. Obviously, additional safety is only useful if efficacy is retained, as suggested by our results here.
A similar approach has also been taken by Bereta et al.; they have generated and used a non oncolytic recombinant vaccinia virus-expressing CD40L to boost the immune system in the context of vaccination.37 In their work, they show that the oncolysate from tumor cells infected with the CD40L-expressing vaccine was effectively capable to activate DCs stimulating secretion of IL12. Similar results have also been presented in study by feder-Mengus et al. that describe a CD40L replicative-deficient vaccinia virus resulted in APC activation thereby enhancing a tumor-specific T-cell response bypassing the requirement of activated T-helper cells.38 All these data, taken together, support our work and give some insights on the mechanism of action of our virus.
In addition to the effect of human CD40L on the immune system, which cannot be tested in mouse experiments due to the species-specificity of CD40L, we observed a certain degree of advantage of the armed virus in CD40-expressing tumors. In accordance with what has been previously suggested, that is, that interaction of CD40L with CD40 can cause apoptosis of tumor cells,11 we have observed an advantage of the armed virus in CD40+ tumor cell line in vivo.
With murine CD40L we saw a significant tumor growth inhibition and prolonged survival in an immunocompetent mouse model. In addition, infiltration of lymphocytes and antigen-presenting cells was detected in the tumors. Although the differences between armed and unarmed virus were not statistically different, we observed a trend that might suggest certain advantage and enhanced immunological responses obtained when tumors were treated with vvdd-tdTomato-mCD40L.
In summary, we have generated and tested a new double-deleted vaccinia virus expressing the CD40L. This virus has increased safety and immunogenicity in addition to have a more pronounced capability to kill CD40-expressing tumors.
Materials and methods
Cell lines
Cancer cell lines used in this study included human breast cancer cell line M4A4-LM,39 lung adenocarcinoma cells A549, human prostate cancer cell line PC3MM2 and african green monkey kidney fibroblasts CV-1 (ATCC, Manassas, VA, USA). EJ cells were provided and authenticated by AG Eliopoulos (University of Crete Medical School and Laboratory of Cancer Biology, Heraklion, Crete, Greece), and murine melanoma cell line B16-ova was provided and authenticated by Richard Vile (Rochester). All cell lines were maintained in the recommended conditions.
All vaccinia viruses used in this study are of the Western Reserve strain with disrupted TK and VGF genes for enhanced cancer cell specificity
For generation of vvdd-tdtomato, the tdTomato gene35 was cloned into pSC65 (a kind gift from Bernie Moss, National Institutes of Health, Bethesda, MD) under the control of the P7.5 promoter to create pSC65-tdTomato. hCD40L or mCD40L cDNA was inserted under the control of the pE/L promoter to create pSC65-tdTomato-CD40l. These shuttle plasmids were co-transfected with vvdd-luc in CV-1 cells. Successfully recombined viruses with tdtomato or with tdtomato-CD40l replaced luciferase from vvdd-luc. New viruses vvdd-tdtomato and vvdd-tdtomato-CD40L were selected by picking plaques positive for red fluorescent and negative for luciferase. Viruses were amplified on A549 cells and purified over a sucrose cushion, and titers were determined with a standard plaque assay on Vero cells as described previously.4 PFU virus titers (PFU/ml) determined by using plaque assay have certain variability and are estimates of the true number of viruses. The presence of the inserted genes was verified by PCR, with fluorescent microscope and with FACSarray from the cells infeced with virus.
UV light inactivation of viruses was done as described before.40 In brief, viruses were suspended in 10 μg ml−1 psoralen in Hanks balanced salt solution containing 0.1% bovine serum albumin. The suspension was incubated for 10 min at room temperature and then irradiated in a CL-1000 UV cross-linker (UVP, Cambridge, UK) with UV-A light (365 nm) for 3 min. A 5-day plaque assay was used to confirm lack of replication-competent virus.
In vitro cytotoxicity assay
Cells on 96-well plates were infected with different concentrations of virus suspended in growth medium containing 2% FCS. One hour later, cells were washed and incubated in growth medium containing 5% FCS for 72 h. Cell viability was then analyzed using MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] (Cell Titer 96 AQueous One Solution proliferation assay; Promega, Madison, WI, USA).
Marker gene transfer assay
Cells on 24-well plates were infected with different concentrations of virus suspended in growth medium containing 2% FCS. Thirty minutes later, cells were washed and incubated in complete growth medium for different time periods. Supernatant was collected and analyzed according to the manufacturer’s manual (FACSarray for soluable hCD40l).
Immunogenicity of cell death
Cells were infected with 1 p.f.u. per cell, and samples were collected after 12 h. For measuring calreticuline exposure, cells were stained with an anticalreticulin antibody, and Alexa-Fluor 488 IgG was used as the secondary antibody for flow cytometry analysis. Extracellular ATP was analyzed with the ATP Determination Kit (A22066, MolecularProbes; Invitrogen, Carlsbad, CA, USA). HMGB1 was measured with the ELISA kit (ST51011; IBLInternational, Hamburg, Germany).
Animal experiments
Animal experiments were approved by the Experimental Animal Committee of the University of Helsinki, Finland. Mice were purchased from Taconic (Ejby, Denmark and Hudson, NY, USA) at the age of 4–5 weeks and housed under standard conditions with food and water ad libitum.
A total of 106 EJ (n=5 mice/group) or A549 (n=5 mice per group) cells were injected subcutaneously into flanks of nude Naval Medical Research Institute (NMRI) mice. When tumors reached the size of approximately 5 by 5 mm, virus was injected either intratumorally (1 × 106 p.f.u.) or intravenously (1 × 107 p.f.u.). Bioluminescence images were captured using the IVIS imaging series 100 system (Xenogen, Alameda, CA, USA).
A total of 2.5 × 105 B16-ova cells (n=10 mice/group) were injected subcutaneously into flanks of immune competent C57Bl/6 mice. When tumors reached the size of approximately 5 by 5 mm, PBS or 107 p.f.u. of virus was injected intratumorally. On day 18, tumors, spleens and lymph nodes were smashed, filtered through a 70-mm filter and cultured for 24 h. Flow cytometric analyses were conducted by flow cytometry according to the manufacturer’s instructions. Simultaneously part of the mice (n=8 mice/group) were monitored in a survival experiment. Animals were euthanized when the tumor size reached 1.5 cm or when the tumor was ulcerating.
FACSarray and flow cytometry
hCD40l concentration in mouse serum and from tumor lysate was determined with FACSarray. To assess hCD40L concentration in blood or in tumor, mice were injected intravenously with virus, and bioluminescent imaging and blood samples were taken 3 and 13 days post injection. On 13 days post injection tumors were collected and lysed with ultrasonification. Samples were analysed with FACSarray for hCD40L quantification.
Cells and tissues from the C57Bl/6 mice bearing B16-ova were stained according to the manufacturer’s instructions with respective antibodies and analyzed with a BD Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Antibodies used for staining were purchased from BD Biosciences: 553066–CD3, 550954–CD4, 553033–CD8, 561736–CD19, 55093–CD11b (Macrophages, MDSC), 553801–CD11c (DC), 560756–NKp46 and 553127–Gr1+ (MDSC). Results were plotted with FacsAccuri C6 software (BD Biosciences).
Human-derived lymphocyte stimulation
A549 cells were infected with different amount of vvdd-tdtomato of vvdd-hCD40l-tdtomato, and supernatant was collected 32h after infection. Supernatant was filtered through x μm filter and added on top of Ramos Blue cells for 24 h stimulation. Ramos-Blue cells are B-lymphocyte cell lines that stably expresses an NFkB/AP-1-inducible SEAP (secreted embryonic alkaline phosphatase) reporter gene. When stimulated, they produce SEAP in the supernatant. The levels of SEAP can be readily monitored using QUANTI-Blue, a medium that turns purple/blue in the presence of SEAP. Levels of activation were read with a microplate reader at the wave length of 450 nm.
Isolated human peripheral blood mononuclear cell stimulation
Confluent 24-well plates of A549 cells were infected with vvdd-tdtomato or vvdd-hCD40L-tdtomato and were allowed to be lysed for 96 h. Samples and control without virus were freeze-thawed two times to completely lyse the cells. Isolated human PBMC (Tebu-bio) were grown as instructed. Lysate were added on top of PBMCs for 16 h, 1 or 3day stimulation; supernatants were analyzed by FACSarray for stimulation markers human TNF alpha, IL-1alpha, IL-6, IL-10 and RANTES.
Statistical analysis
Tumor sizes as a function of time were compared by Mann–Whitney U-test (SPSS 16.0; SPSS Inc., Chicago, IL, USA), and P-values of <0.05 were considered statistically significant. A single preplanned comparison of mean tumor volume was done by using a two-tailed t-test. Fot the data of survival experiments, a log-rank t-test was used for pairwise comparison of groups.
References
Symons JA, Alcami A, Smith GL . Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 1995; 81: 551–560.
Alcami A, Smith GL . Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J Virol 1995; 69: 4633–4639.
Colamonici OR, Domanski P, Sweitzer SM, Larner A, Buller RM . Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J Biol Chem 1995; 270: 15974–15978.
McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res 2001; 61: 8751–8757.
Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest 2007; 117: 3350–3358.
Trinchieri G, Aden DP, Knowles BB . Cell-mediated cytotoxicity to SV40-specific tumour-associated antigens. Nature 1976; 261: 312–314.
Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol 2003; 170: 4905–4913.
Grewal IS, Flavell RA . CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 1998; 16: 111–135.
Eliopoulos AG, Dawson CW, Mosialos G, Floettmann JE, Rowe M, Armitage RJ et al. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr Virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene 1996; 13: 2243–2254.
Tong AW, Papayoti MH, Netto G, Armstrong DT, Ordonez G, Lawson JM et al. Growth-inhibitory effects of CD40 ligand (CD154) and its endogenous expression in human breast cancer. Clin Cancer Res 2001; 7: 691–703.
Eliopoulos AG, Davies C, Knox PG, Gallagher NJ, Afford SC, Adams DH et al. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol Cell Biol 2000; 20: 5503–5515.
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331: 1612–1616.
von Euler H, Sadeghi A, Carlsson B, Rivera P, Loskog A, Segall T et al. Efficient adenovector CD40 ligand immunotherapy of canine malignant melanoma. J Immunother 2008; 31: 377–384.
Iida T, Shiba H, Misawa T, Ohashi T, Eto Y, Yanaga K . Adenovirus-mediated CD40L gene therapy induced both humoral and cellular immunity against rat model of hepatocellular carcinoma. Cancer Sci 2008; 99: 2097–2103.
Meziane el K, Bhattacharyya T, Armstrong AC, Qian C, Hawkins RE, Stern PL et al. Use of adenoviruses encoding CD40L or IL-2 against B cell lymphoma. Int J Cancer 2004; 111: 910–920.
Pesonen S, Diaconu I, Kangasniemi L, Ranki T, Kanerva A, Pesonen SK et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res 2012; 72: 1621–1631.
Diaconu I, Cerullo V, Hirvinen ML, Escutenaire S, Ugolini M, Pesonen SK et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res 2012; 72: 2327–2338.
Tomihara K, Kato K, Masuta Y, Nakamura K, Tanaka T, Hiratsuka H et al. Gene transfer of the CD40-ligand to human dendritic cells induces NK-mediated antitumor effects against human carcinoma cells. Int J Cancer 2007; 120: 1491–1498.
Gonzalez-Carmona MA, Lukacs-Kornek V, Timmerman A, Shabani S, Kornek M, Vogt A et al. CD40ligand-expressing dendritic cells induce regression of hepatocellular carcinoma by activating innate and acquired immunity in vivo. Hepatology 2008; 48: 157–168.
Wiethe C, Dittmar K, Doan T, Lindenmaier W, Tindle R . Enhanced effector and memory CTL responses generated by incorporation of receptor activator of NF-kappa B (RANK)/RANK ligand costimulatory molecules into dendritic cell immunogens expressing a human tumor-specific antigen. J Immunol 2003; 171: 4121–4130.
Zitvogel L, Kepp O, Galluzzi L, Kroemer G . Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol 2012; 13: 343–351.
Caux C, Zitvogel L . Recent successes of cancer immunotherapy: a new dimension in personalized medicine? Targeted Oncol 2012; 7: 1–2.
Galluzzi L, Senovilla L, Zitvogel L, Kroemer G . The secret ally: immunostimulation by anticancer drugs. Nature reviews Drug discovery 2012; 11: 215–233.
Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ 2008; 15: 1499–1509.
Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007; 220: 47–59.
Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res 2010; 70: 855–858.
Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 2011; 477: 99–102.
Kirn DH, Thorne SH . Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer 2009; 9: 64–71.
Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol 2008; 9: 533–542.
Winnard PT Jr, Kluth JB, Raman V . Noninvasive optical tracking of red fluorescent protein-expressing cancer cells in a model of metastatic breast cancer. Neoplasia 2006; 8: 796–806.
Shaner NC, Lin MZ, McKeown MR, Steinbach PA, Hazelwood KL, Davidson MW et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat Methods 2008; 5: 545–551.
Ruby J, Bluethmann H, Aguet M, Ramshaw IA . CD40 ligand has potent antiviral activity. Nat Med 1995; 1: 437–441.
Hannani D, Sistigu A, Kepp O, Galluzzi L, Kroemer G, Zitvogel L . Prerequisites for the antitumor vaccine-like effect of chemotherapy and radiotherapy. Cancer J 2011; 17: 351–358.
Guo ZS, Naik A, O'Malley ME, Popovic P, Demarco R, Hu Y et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res 2005; 65: 9991–9998.
Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY . Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 2004; 22: 1567–1572.
Le Boeuf F, Diallo JS, McCart JA, Thorne S, Falls T, Stanford M et al. Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther 2010; 18: 888–895.
Bereta M, Bereta J, Park J, Medina F, Kwak H, Kaufman HL . Immune properties of recombinant vaccinia virus encoding CD154 (CD40L) are determined by expression of virally encoded CD40L and the presence of CD40L protein in viral particles. Cancer Gene Ther 2004; 11: 808–818.
Feder-Mengus C, Schultz-Thater E, Oertli D, Marti WR, Heberer M, Spagnoli GC et al. Nonreplicating recombinant vaccinia virus expressing CD40 ligand enhances APC capacity to stimulate specific CD4+ and CD8+ T cell responses. Hum Gene Ther 2005; 16: 348–360.
Ranki T, Sarkioja M, Hakkarainen T, von Smitten K, Kanerva A, Hemminki A . Systemic efficacy of oncolytic adenoviruses in imagable orthotopic models of hormone refractory metastatic breast cancer. Int J Cancer 2007; 121: 165–174.
Tsung K, Yim JH, Marti W, Buller RM, Norton JA . Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J Virol 1996; 70: 165–171.
Acknowledgements
This work was supported by Helsinki Biomedical Graduate School, Helsinki Graduate Program in Biotechnology and Molecular Biology, University of Helsinki postdoctoral grant, European Research Council, HUCH Research Funds (EVO), Finnish Cancer Society, Sigrid Juselius Foundation, Academy of Finland, University of Helsinki, Marie Curie FP7-IRG-2008, K Albin Johansson Foundation, Biocentrum Helsinki, Biocenter Finland, University of Helsinki and ASCO Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
AH is shareholder in and paid consultant for Oncos Therapeutics, Ltd. AH is shareholder in TILT Biotherapeutics Ltd. The remaining authors declare no conflict of interest
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Parviainen, S., Ahonen, M., Diaconu, I. et al. CD40 ligand and tdTomato-armed vaccinia virus for induction of antitumor immune response and tumor imaging. Gene Ther 21, 195–204 (2014). https://doi.org/10.1038/gt.2013.73
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.73
Keywords
This article is cited by
-
Colonization of xenograft tumors by oncolytic vaccinia virus (VACV) results in enhanced tumor killing due to the involvement of myeloid cells
Journal of Translational Medicine (2016)
-
Expression of DAI by an oncolytic vaccinia virus boosts the immunogenicity of the virus and enhances antitumor immunity
Molecular Therapy - Oncolytics (2016)
-
Targeted therapies in bladder cancer: an overview of in vivo research
Nature Reviews Urology (2015)
-
Safety and biodistribution of a double-deleted oncolytic vaccinia virus encoding CD40 ligand in laboratory Beagles
Molecular Therapy - Oncolytics (2014)
-
Potentiation of immunomodulatory antibody therapy with oncolytic viruses for treatment of cancer
Molecular Therapy - Oncolytics (2014)