Abstract
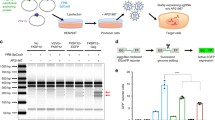
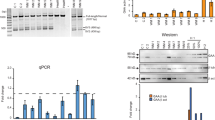
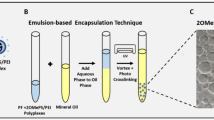
A series of small-size polyethylenimine (PEI)-conjugated pluronic polycarbamates (PCMs) have been investigated for the ability to modulate the delivery of 2′-O-methyl phosphorothioate RNA (2′-OMePS) in vitro and in dystrophic mdx mice. The PCMs retain strong binding capacity to negatively charged oligomer as demonstrated by agarose gel retardation assay, with the formation of condensed polymer/oligomer complexes at a wide-range weight ratio from 1:1 to 20:1. The condensed polymer/oligomer complexes form 100–300 nm nanoparticles. Exon-skipping effect of 2′-OMePS was dramatically enhanced with the use of the most effective PCMs in comparison with 2′-OMePS alone in both cell culture and in vivo, respectively. More importantly, the effective PCMs, especially those composed of moderate size (2k–5kDa) and intermediate hydrophilic–lipophilic balance (7–23) of pluronics, enhanced exon-skipping of 2′-OMePS with low toxicity as compared with Lipofectamine-2000 in vitro or PEI 25k in vivo. The variability of individual PCM for delivery of antisense oligomer and plasmid DNA indicate the complexity of interaction between polymer and their cargos. Our data demonstrate the potential of PCMs to mediate delivery of modified antisense oligonucleotides to the muscle for treating muscular dystrophy or other appropriate myodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hoffman EP, Brown RH Jr, Kunkel LM . Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51: 919–928.
Koenig M, Beggs AH, Moyer M, Scherpf S, Heindrich K, Bettecken T et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet 1989; 45: 498–506.
Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Spurney CF et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA 2008; 105: 14814–14819.
Yin H, Moulton HM, Seow Y, Boyd C, Boutilier J, Iverson P et al. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet 2008; 17: 3909–3918.
Wu B, Lu P, Benrashid E, Malik S, Ashar J, Doran TJ et al. Dose-dependent restoration of dystrophin expression in cardiac muscle of dystrophic mice by systemically delivered morpholino. Gene Ther 2009; 17: 132–140.
Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, Heuvelmans N et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med 2011; 364: 1513–1522.
Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, Anthony K et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 2011; 378: 595–605.
Sirsi SR, Schray RC, Guan X, Lykens NM, Williams JH, Erney ML et al. Functionalized PEG-PEI copolymers complexed to exon-skipping oligonucleotides improve dystrophin expression in mdx mice. Hum Gene Ther 2008; 19: 795–806.
Dubowitz V . A short history of the World Muscle Society. Neuromuscul Disord 2005; 15: 642–647.
Partridge TA . Stem cell route to neuromuscular therapies. Muscle Nerve 2003; 27: 133–141.
Wagner KR, Lechtzin N, Judge DP . Current treatment of adult Duchenne muscular dystrophy. Biochim Biophys Acta 2007; 1772: 229–237.
Townsend D, Yasuda S, Li S, Chamberlain JS, Metzger JM . Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther 2008; 16: 832–835.
Williams JH, Sirsi SR, Latta DR, Lutz GJ . Induction of dystrophin expression by exon-skipping in mdx mice following intramuscular injection of antisense oligonucleotides complexed with PEG-PEI copolymers. Mol Ther 2006; 14: 88–96.
Koo T, Wood. MJ . Clinical trials using antisense oligonucleotides in Duchenne muscular dystrophy. Hum Gene Ther 2013; 24: 479–488.
Eckstein F . Phosphorothioate oligodeoxynucleotides: what is their origin and what is unique about them? Antisense Nucleic Acid Drug Dev 2000; 10: 117–121.
Kurreck J . Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem 2003; 270: 1628–1644.
Chiappetta DA, Sosnik A . Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharm 2007; 66: 303–317.
Sriadibhatla S, Yang Z, Gebhart C, Alakhov VY, Kabanov A . Transcriptional activation of gene expression by pluronic block copolymers in stably and transiently transfected cells. Mol Ther 2006; 13: 804–813.
Pitard B, Pollard H, Agbulut O, Lambert O, Vilquin JT, Cherel Y et al. A nonionic amphiphile agent promotes gene delivery in vivo to skeletal and cardiac muscles. Hum Gene Ther 2002; 13: 1767–1775.
Roques C, Fromes Y, Fattal E . Hydrosoluble polymers for muscular gene delivery. Eur J Pharm Biopharm 2009; 72: 378–390.
Richard P, Bossard F, Desigaux L, Lanctin C, Bello-Roufai M, Pitard B . Amphiphilic block copolymers promote gene delivery in vivo to pathological skeletal muscles. Hum Gene Ther 2005; 16: 1318–1324.
Boussif O, Lezoualch F, Zanta MA, Mergny MD, Scherman D, Demeneix B et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad. Sci. USA 1995; 92: 7297–7301.
Lungwitz U, Breunig M, Blunk T, Gopferich A . Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm 2005; 60: 247–266.
Choosakoonkriang S, Lobo BA, Koe GS, Koe JG, Middaugh CR . Biophysical characterization of PEI/DNA complexes. J Pharm Sci 2003; 92: 1710–1722.
Garrett SW, Davies OR, Milroy DA, Wood PJ, Pouton CW, Threadgill MD . Synthesis and characterisation of polyamine-poly(ethylene glycol) constructs for DNA binding and gene delivery. Bioorg Med Chem 2000; 8: 1779–1797.
Gebhart CL, Sriadibhatla S, Vinogradov S, Lemieux P, Alakhov V, Kabanov AV . Design and formulation of polyplexes based on pluronic-polyethyleneimine conjugates for gene transfer. Bioconjug Chem 2002; 13: 937–944.
Wang MX, Lu P, Wu B, Tucker JD, Cloer C, Lu QL . High efficiency and low toxicity of polyethyleneimine modified pluronics (PEI-Pluronic) as gene delivery carriers in cell culture and dystrophic mdx mice. J Mater Chem 2012; 22: 6038–6046.
Wang MX, Wu B, Lu PJ, Cloer C, Tucker JD, Lu QL . Polyethylenimine modified pluronics (PCMs) improve morpholino oligomer delivery in cell culture and dystrophic mdx mice. Mol Ther 2013; 21: 210–216.
Grayson AC, Doody AM, Putnam D . Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm Res 2006; 23: 1868–1876.
Jääskeläinen I, Peltola S, Honkakoski P, Mönkkönen J, Urtti A . A lipid carrier with a membrane active component and a small complex size are required for efficient cellular delivery of anti-sense phosphorothioate oligonucleotides. Eur J Pharm Sci 2000; 10: 187–193.
Eliyahu H, Barenholz Y, Domb AJ . Polymers for DNA delivery. Molecules 2005; 10: 34–64.
Patrick M, Chantal P, Jean-Jacques Y, Paul-Alain J . Chemical vectors for gene delivery: a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br J Pharmacol 2009; 157: 166–178.
Roques C, Bouchemal K, Ponchel G, Yves Fromes Y, Elias Fattal E . Parameters affecting organization and transfection efficiency of amphiphilic copolymers/DNA carriers. J Control Release 2009; 138: 71–77.
Sharma VK, Thomas M, Klibanov AM . Mechanistic studies on aggregation of polyethylenimine-DNA complexes and its prevention. Biotechnol Bioeng 2005; 90: 614–620.
Rimessi P, Sabatelli P, Fabris M, Braghetta P, Bassi E, Spitali P et al. Cationic PMMA nanoparticles bind and deliver antisense oligoribonucleotides allowing restoration of dystrophin expression in the mdx mouse. Mol Ther 2009; 17: 820–827.
Acknowledgements
We thank Dr Craig A Ogle at the Department of Chemistry, University of North Carolina, Charlotte, for guidance in measuring 1H-NMR and DLS. We also thank Ms Daisy Ridings and Mr David Radoff for TEM analysis, Dr David M Foureau for fluorescence-activated cell sorting analysis. We also gratefully acknowledge the Carolinas Muscular Dystrophy Research Endowment at the Carolinas HealthCare Foundation and Carolinas Medical Center, Charlotte, NC, USA.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, M., Wu, B., Lu, P. et al. Pluronic–PEI copolymers enhance exon-skipping of 2′-O-methyl phosphorothioate oligonucleotide in cell culture and dystrophic mdx mice. Gene Ther 21, 52–59 (2014). https://doi.org/10.1038/gt.2013.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.57
Keywords
This article is cited by
-
Peptide-Mediated Tumor Targeting by a Degradable Nano Gene Delivery Vector Based on Pluronic-Modified Polyethylenimine
Nanoscale Research Letters (2016)
-
Poly(ester amine) Composed of Polyethylenimine and Pluronic Enhance Delivery of Antisense Oligonucleotides In Vitro and in Dystrophic mdx Mice
Molecular Therapy - Nucleic Acids (2016)
-
Correlating In Vitro Splice Switching Activity With Systemic In Vivo Delivery Using Novel ZEN-modified Oligonucleotides
Molecular Therapy - Nucleic Acids (2014)