Abstract
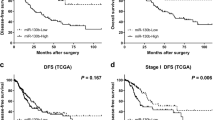
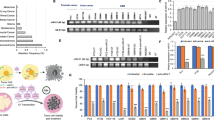
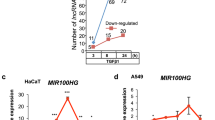
MicroRNA dysregulation often results in the development and progression of cancer. miR-143 is ubiquitously expressed in most human and murine tissues but downregulated in many cancer types. This differential miRNA expression can be utilized for targeted cancer gene therapies. Multiple copies of the miR-143 complementary target sequence were inserted into the 3′UTR of plasmid vectors encoding either for different reporter genes or for the therapeutic gene TNFα. With these transgenes, we analyzed the miR-143-dependent gene expression in cancer cells and normal cells. Moreover, we investigated miR-143-regulated luciferase expression in an NMRI nude/HUH7 xenograft mouse model using a nonviral carrier system for in vivo transfections. We showed low and high levels of miR-143 in cancer cells and normal cells, respectively, leading to a differential gene expression of the reporters and the therapeutic TNFα. According to the miR-143 levels, the luciferase reporter gene expression was silenced in the mouse lungs but not in HUH7 tumors. Thus, we utilized the differential miR-143 expression in healthy and cancerous tissues to de-target the lung by specifically targeting the tumor in an in vivo HUH7 xenograft mouse model. The use of an miR-143-regulated therapeutic transgene may present a promising approach for cancer gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297.
Friedman RC, Farh KK, Burge CB, Bartel DP . Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009; 19: 92–105.
Iorio MV, Croce CM . MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med 2012; 4: 143–159.
Kahlert C, Klupp F, Brand K, Lasitschka F, Diederichs S, Kirchberg J et al. Invasion front-specific expression and prognostic significance of microRNA in colorectal liver metastases. Cancer Sci 2011; 102: 1799–1807.
Pichler M, Winter E, Stotz M, Eberhard K, Samonigg H, Lax S et al. Down-regulation of KRAS-interacting miRNA-143 predicts poor prognosis but not response to EGFR-targeted agents in colorectal cancer. Br J Cancer 2012; 106: 1826–1832.
Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y . Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology 2009; 77: 12–21.
Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene 2009; 28: 1385–1392.
Kent OA, Fox-Talbot K, Halushka MK. . RREB1 repressed miR-143/145 modulates KRAS signaling through downregulation of multiple targets. Oncogene 2013; 32: 2576–2585.
Borralho PM, Kren BT, Castro RE, da Silva IB, Steer CJ, Rodrigues CM . MicroRNA-143 reduces viability and increases sensitivity to 5-fluorouracil in HCT116 human colorectal cancer cells. FEBS J 2009; 276: 6689–6700.
Kulda V, Pesta M, Topolcan O, Liska V, Treska V, Sutnar A et al. Relevance of miR-21 and miR-143 expression in tissue samples of colorectal carcinoma and its liver metastases. Cancer Genet Cytogenet 2010; 200: 154–160.
Zhang Y, Wang Z, Chen M, Peng L, Wang X, Ma Q et al. MicroRNA-143 targets MACC1 to inhibit cell invasion and migration in colorectal cancer. Mol Cancer 2012; 11: 23.
Peng X, Guo W, Liu T, Wang X, Tu X, Xiong D et al. Identification of miRs-143 and -145 that is associated with bone metastasis of prostate cancer and involved in the regulation of EMT. Plos One 2011; 6: e20341.
Borralho PM, Simoes AE, Gomes SE, Lima RT, Carvalho T, Ferreira DM et al. miR-143 overexpression impairs growth of human colon carcinoma xenografts in mice with induction of apoptosis and inhibition of proliferation. Plos ONE 2011; 6: e23787.
Pramanik D, Campbell NR, Karikari C, Chivukula R, Kent OA, Mendell JT et al. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther 2011; 10: 1470–1480.
Lee CY, Rennie PS, Jia WW . MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin Cancer Res 2009; 15: 5126–5135.
Cawood R, Chen HH, Carroll F, Bazan-Peregrino M, van Rooijen N, Seymour LW . Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog 2009; 5: e1000440.
Edge RE, Falls TJ, Brown CW, Lichty BD, Atkins H, Bell JC . A let-7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther 2008; 16: 1437–1443.
Kelly EJ, Nace R, Barber GN, Russell SJ . Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J Virol 2010; 84: 1550–1562.
Perez JT, Pham AM, Lorini MH, Chua MA, Steel J, tenOever BR . MicroRNA-mediated species-specific attenuation of influenza A virus. Nat Biotechnol 2009; 27: 572–576.
Ylosmaki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R, Saksela K . Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol 2008; 82: 11009–11015.
Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L . Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med 2006; 12: 585–591.
Kamrud KI, Coffield VM, Owens G, Goodman C, Alterson K, Custer M et al. In vitro and in vivo characterization of microRNA-targeted alphavirus replicon and helper RNAs. J Virol 2010; 84: 7713–7725.
Lee TC, Lin YL, Liao JT, Su CM, Lin CC, Lin WP et al. Utilizing liver-specific microRNA-122 to modulate replication of dengue virus replicon. Biochem Biophys Res Commun 2010; 396: 596–601.
Suzuki T, Sakurai F, Nakamura S, Kouyama E, Kawabata K, Kondoh M et al. miR-122a-regulated expression of a suicide gene prevents hepatotoxicity without altering antitumor effects in suicide gene therapy. Mol Ther 2008; 16: 1719–1726.
Rapp UR, Korn C, Ceteci F, Karreman C, Luetkenhaus K, Serafin V et al. MYC is a metastasis gene for non-small-cell lung cancer. Plos ONE 2009; 4: e6029.
Magnusson T, Haase R, Schleef M, Wagner E, Ogris M . Sustained, high transgene expression in liver with plasmid vectors using optimized promoter-enhancer combinations. J Gene Med 2011; 13: 382–391.
Su B, Cengizeroglu A, Farkasova K, Viola JR, Anton M, Ellwart JW et al. Systemic TNFalpha gene therapy synergizes with liposomal doxorubicine in the treatment of metastatic cancer. Mol Ther 2013; 21: 300–308.
Russ V, Elfberg H, Thoma C, Kloeckner J, Ogris M, Wagner E . Novel degradable oligoethylenimine acrylate ester-based pseudodendrimers for in vitro and in vivo gene transfer. Gene Ther 2008; 15: 18–29.
Smrekar B, Wightman L, Wolschek MF, Lichtenberger C, Ruzicka R, Ogris M et al. Tissue-dependent factors affect gene delivery to tumors in vivo. Gene Ther 2003; 10: 1079–1088.
Klutz K, Schaffert D, Willhauck MJ, Grunwald GK, Haase R, Wunderlich N et al. Epidermal growth factor receptor-targeted (131)i-therapy of liver cancer following systemic delivery of the sodium iodide symporter gene. Mol Ther 2011; 19: 676–685.
Schaffert D, Troiber C, Salcher EE, Frohlich T, Martin I, Badgujar N et al. Solid-phase synthesis of sequence-defined T-, i-, and U-shape polymers for pDNA and siRNA delivery. Angew Chem Int Ed Engl 2011; 50: 8986–8989.
Ogris M, Wagner E . To be targeted: is the magic bullet concept a viable option for synthetic nucleic acid therapeutics? Hum Gene Ther 2011; 22: 799–807.
Peltier HJ, Latham GJ . Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA 2008; 14: 844–852.
Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP . Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 2007; 3: 12.
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 2005; 33: e179.
Haase R, Argyros O, Wong SP, Harbottle RP, Lipps HJ, Ogris M et al. pEPito: a significantly improved non-viral episomal expression vector for mammalian cells. BMC Biotechnol 2010; 10: 20.
Navarro G, Maiwald G, Haase R, Rogach AL, Wagner E, de Ilarduya CT et al. Low generation PAMAM dendrimer and CpG free plasmids allow targeted and extended transgene expression in tumors after systemic delivery. J Control Release 2010; 146: 99–105.
Acknowledgements
The authors thank Professor Ulf R Rapp and Dr Chitra Thakur for providing material from the SpC-c-MYC transgenic mouse model and Dr Andreas Wieser for providing the pRVmCherry construct. Moreover, we would like to thank Dr Arzu Cengizeroglu for performing the TNFα ELISA. This work was supported by the DFG grant no. OG 63/4–1 (M.O.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Kopp, F., Schnoedt, M., Haase, R. et al. De-targeting by miR-143 decreases unwanted transgene expression in non-tumorigenic cells. Gene Ther 20, 1104–1109 (2013). https://doi.org/10.1038/gt.2013.37
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.37
Keywords
This article is cited by
-
Improved sugar recovery from enzymatic hydrolysis of Miscanthus sinensis by surfactant-mediated alkaline pretreatment
Biomass Conversion and Biorefinery (2023)
-
Cellular and viral microRNAs in sepsis: mechanisms of action and clinical applications
Cell Death & Differentiation (2016)