Abstract
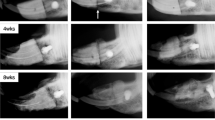
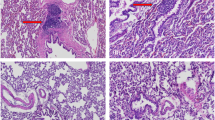
Nonhealing bone defects are difficult to treat. As the bone morphogenic protein and transforming growth factor beta pathways have been implicated in bone healing, we hypothesized that percutaneous Smad7 silencing would enhance signaling through both pathways and improve bone formation. Critical sized parietal trephine defects were created and animals received percutaneous injection of: agarose alone or agarose containing nonsense or Smad7 small interfering RNA (siRNA). At 12 weeks, SMADs1, 2, 3, 5, 7 and 8 levels were assessed. Smad1/5/8 osteogenic target, Dlx5, and SMAD2/3 angiogenic target, plasminogen activator inhibitor-1 (Pai1), transcription levels were measured. Noncanonical signaling through TGFβ activated kinase-1 (Tak1) and target, runt-related transcription factor 2 (Runx2) and collagen1α1 (Col1α1), transcription were also measured. Micro-computed tomography and Gomori trichome staining were used to assess healing. Percutaneous injection of Smad7 siRNA significantly knocked down Smad7 mRNA (86.3±2.5%) and protein levels (46.3±3.1%). The SMAD7 knockdown resulted in a significant increase in receptor-regulated SMADs (R-SMAD) (Smad 1/5/8 and Smad2/3) nuclear translocation. R-SMAD nuclear translocation increased Dlx5 and Pai1 transcription. Additionally, noncanonical signaling through Tak1 increased Runx2 and Col1α1 target transcription. Compared with animals treated with agarose alone (33.9±2.8% healing) and nonsense siRNA (31.5±11.8% healing), animals treated Smad7 siRNA had significantly great (91.2±3.8%) healing. Percutaneous Smad7 silencing increases signal transduction through canonical and noncanonical pathways resulting in significant bone formation. Minimally invasive gene therapies may prove effective in the treatment of nonhealing bone defects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Warren S, Fong K, Chen C, Loboa E, Cowan C, Lorenz H et al. Tools and techniques for craniofacial tissue engineering. Tissue Eng 2003; 9: 187–200.
Szpalski C, Barr J, Wetterau M, Saadeh P, Warren S . Cranial bone defects: current and future strategies. Neurosurg Focus 2010; 29: E8.
Bhumiratana S, Vunjak-Novakovic G . Concise review: personalized human bone grafts for reconstructing head and face. Stem Cells Transl Med 2012; 1: 64–69.
Tadros M, Costantino P . Advances in cranioplasty: a simplified algorithm to guide cranial reconstruction of acquired defects. Facial Plast Surg 2008; 24: 135–145.
Massagué J, Seoane J, Wotton D . Smad transcription factors. Genes Dev 2005; 19: 2783–2810.
Massagué J . Tgf-beta signal transduction. Annu Rev Biochem 1998; 67: 753–791.
Nakao A, Afrakhte M, Morén A, Nakayama T, Christian J, Heuchel R et al. Identification of smad7, a tgfβ-inducible antagonist of tgf-β signaling. Nature 1997; 389: 631–635.
Hayashi H, Abdollah S, Qiu Y, Cai J, Xu Y, Grinnell B et al. The mad-related protein smad7 associates with the tgfβ receptor and functions as an antagonist of tgfβ signaling. Cell 1997; 89: 1165–1173.
Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M et al. Smad6 inhibits signaling by the tgf-β superfamily. Nature 1997; 389: 622–626.
Hata A, Lagna G, Massagué J, Hemmati-Brivanlou A . Smad6 inhibits bmp/smad1 signaling by specifically competing with the smad4 tumor suppressor. Genes Dev 1998; 12: 186–197.
Reddi A . Bone morphogenetic proteins: from basic science to clinical applications. J Bone Joint Surg Am 2001; 1: S1–S6.
Kawabata M, Imamura T, Miyazono K . Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev 1998; 9: 49–61.
Koh J, Zhao Z, Wang Z, Lewis I, Krebsbach PH, Francesch R . Combinatorial gene therapy with bmp2/7 enhances cranial bone regeneration. J Dent Res 2008; 87: 845–849.
Yarnold J, Brotons M . Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol 2010; 97: 149–161.
Sakou T, Onishi T, Yamamoto T, Nagamine T, Sampath T, Ten Dijke P . Localization of smads, the tgf-beta family intracellular signaling components during endochondral ossification. J Bone Min Res 1999; 14: 1145–1152.
Janssens K, ten Dijke P, Janssens S, Van Hul W . Transforming growth factor-beta1 to the bone. Endocr Rev 2005; 26: 743–774.
Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K . Smad3 promotes alkaline phosphatase activity and mineralization of osteoblastic mc3t3-e1 cells. J Bone Min Res 2002; 17: 1190–1199.
Borton A, Frederick J, Datto M, Wang X, Weinstein R . The loss of smad3 results in a lower rate of bone formation and osteopenia through dysregulation of osteoblast differentiation and apoptosis. J Bone Miner Res 2001; 16: 1754–1764.
Greenblatt M, Shim J, Zou W, Sitara D, Schweitzer M, Hu D . The p38 mapk pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest 2010; 120: 2457–2473.
Kim S, Kwak J, Zachariah M, He Y, Wang L, Choi M . Tgf-beta-activated kinase 1 and tak1-binding protein 1 cooperate to mediate tgf-beta1-induced mkk3-p38 mapk activation and stimulation of type i collagen. Am J Physiol Renal Physiol 2007; 292: 1471–1478.
Li H, Daculsi R, Bareille R, Bourget C, Amedee J . Upa and mmp-2 were involved in self-assembled network formation in a two dimensional co-culture model of bone marrow stromal cells and endothelial cells. J Cell Biochem 2013; 114: 650–657.
Ebrahimian T, Squiban C, Roque T, Lugo-Martinez H, Hneino M, Buard V et al. Plasminogen activator inhibitor-1 controls bone marrow-derived cells therapeutic effect through mmp9 signaling: role in physiological and pathological wound healing. Stem Cells 2012; 30: 1436–1446.
Zhang J, Lei T, Chen X, Peng Y, Long H, Zhou L et al. Resistin up-regulates cox-2 expression via tak1-ikk-nf-kappab signaling pathway. Inflammation 2010; 33: 25–33.
Wang X, Allen RJ, Tutela J, Sailon A, Allor iA, Davidson E et al. Progenitor cell mobilization enhances bone healing by means of improved neovascularization and osteogenesis. Plast Reconstr Surg 2011; 128: 395–405.
Behr B, Sorkin M, Lehnhardt M, Renda A, Longaker MT, Quarto N . A comparative analysis of the osteogenic effects of bmp-2, fgf-2, and vegfa in a calvarial defect model. Tissue Eng Part A 2012; 18: 1079–1086.
Sultan SM, Davidson EH, Butala P, Schachar JS, Witek L, Szpalski C et al. Interval cranioplasty: comparison of current standards. Plast Reconstr Surg 2011; 127: 1855–1864.
Wrana J, Attisano L, Wieser R, Ventura F, Massagué J . Mechanism of activation of the tgf-β receptor. Nature 1994; 370: 341–347.
Ishisaki A, Yamato K, Nakao A, Nonaka K, Ohguchi M, ten Dijke P et al. Smad7 is an activininducible inhibitor of activin-induced growth arrest and apoptosis in mouse b cells. J Biol Chem 1998; 273: 24293–24296.
Benchabane H, Wrana J . Gata- and smad1-dependent enhancers in the smad7 gene differentially interpret bone morphogenetic protein concentrations. Mol Cell Biol 2003; 23: 6646–6661.
Kavsak P, Rasmussen R, Causing C, Bonni S, Zhu H, Thomsen G et al. Smad7 binds to smurf2 to form an e3 ubiquitin ligase that targets the tgfβ receptor for degradation. Mol Cell Biol 2000; 6: 1365–1375.
Suzuk iC, Murakami G, Fukuchi M, Shimanuki T, Shikauchi Y, Imamura T et al. Smurf1 regulates the inhibitory activity of smad7 by targeting smad7 to the plasma membrane. J Biol Chem 2002; 277: 39919–39925.
Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T . Cooperative inhibition of bone morphogenetic protein signaling by smurf1 and inhibitory smads. Mol Biol Cell 2003; 14: 2809–2817.
Tajima Y, Goto K, Yoshida M, Shinomiya K, Sekimoto T, Yoneda Y et al. Chromosomal region maintenance 1 (crm1) dependent nuclear export of smad ubiquitin regulatory factor 1 (smurf1) is essential for negative regulation of transforming growth factor-β signaling by smad7. J Biol Chem 2003; 278: 10716–10721.
Huse M, Muir T, Xu L, Chen Y, Kuriyan J, Massagué J . The tgf beta receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell Biol 2001; 8: 671–682.
Shi Y, Massagué J . Mechanisms of tgf-beta signaling from cell membrane to the nucleus. Cell 2003; 113: 685–700.
Xu L, Massagué J . Nucleocytoplasmic shuttling of signal transducers. Nat Rev Mol Cell Biol 2004; 5: 209–219.
Inman G, Hill C . Stoichiometry of active smad-transcription factor complexes on DNA. J Biol Chem 2002; 277: 51008–51016.
Greenblatt M, Shim J, Glimcher L . Tak1 mediates bmp signaling in cartilage. Ann N Y Acad Sc 2010; 1192: 385–390.
Afzal F, Pratap J, Ito K, Ito Y, Stein J, van Wijnen A . Smad function and intranuclear targeting share a runx2 motif required for osteogenic lineage induction and bmp2 responsive transcription. J Cell Physiol 2005; 204: 63–72.
Castano-Izquierdo H, Alvarez-Barreto J, van den Dolder J, Jansen JA, Mikos AG, Sikavitsas VI . Pre-culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A 2007; 82: 129–138.
Petrie Aronin CE, Sadik KW, Lay AL, Rion DB, Tholpady SS, Ogle RC et al. Comparative effects of scaffold pore size, pore volume, and total void volume on cranial bone healing patterns using microsphere-based scaffolds. J Biomed Mater Res A 2009; 89: 632–641.
Srouji S, Ben-David D, Lotan R, Livne E, Avrahami R, Zussman E . Slow-release human recombinant bone morphogenetic protein-2 embedded within electrospun scaffolds for regeneration of bone defect: in vitro and in vivo evaluation. Tissue Eng Part A 2011; 17: 269–277.
Vigier S, Catania C, Baroukh B, Saffar JL, Giraud-Guille MM, Colombier ML . Dense fibrillar collagen matrices sustain osteoblast phenotype in vitro and promote bone formation in rat calvaria defect. Tissue Eng Part A 2011; 17: 889–898.
Szpalski C, Nguyen PD, Cretiu Vasiliu CE, Chesnoiu-Matei I, Ricci JL, Clark E et al. Bony engineering using time-release porous scaffolds to provide sustained growth factor delivery. J Craniofac Surg 2012; 23: 638–644.
Thanik V, Greives M, Lerman O, Seiser N, Dec W, Chang C et al. Topical matrix-based sirna silences local gene expression in a murine wound model. Gene Ther 2007; 14: 1305–1308.
Lee J, Tutela J, Zoumalan R, Thanik V, Nguyen P, Varjabedian L et al. Inhibition of smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch Otolaryngol Head Neck Surg 2010; 136: 714–719.
Corporation I. Agarose ultra pure, material safety data sheet Invitrogen Corporation. Carlsbad: CA, USA, 2005.
Jiang M, Rubbi C, Milner J . Gel-based application of sirna to human epithelial cancer cells induces rnai-dependent apoptosis. Oligonucleotides 2004; 14: 239–248.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented at the Northeastern Society of Plastic Surgeons 28th Annual Meeting, October 2011, Amelia Island, FL, USA. American Society of Plastic Surgery Annual Meeting September 2011, Denver, CO, USA. Awards: Outstanding Paper Presentation Award at the American Society of Plastic Surgery Annual Meeting, 2011 (awarded to JL)
Author contributions
JL and FS: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing and final approval of manuscript; AW: collection and/or assembly of data, data analysis and interpretation and manuscript writing; AM, CS: collection and/or assembly of data and data analysis and interpretation; PBS, SMW: conception and design and final approval of manuscript.
Rights and permissions
About this article
Cite this article
Layliev, J., Sagebin, F., Weinstein, A. et al. Percutaneous gene therapy heals cranial defects. Gene Ther 20, 922–929 (2013). https://doi.org/10.1038/gt.2013.15
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.15
Keywords
This article is cited by
-
BMP-2 Induced Signaling Pathways and Phenotypes: Comparisons Between Senescent and Non-senescent Bone Marrow Mesenchymal Stem Cells
Calcified Tissue International (2022)