Abstract
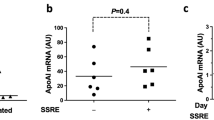
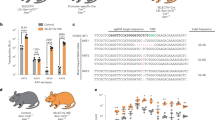
Targeting diseased cells is a challenging issue in both pharmacological and biological therapeutics. Gene therapy is emerging as a novel approach for treating rare diseases and for illnesses for which there is no other alternative. An important limitation of gene therapy has been the off-target effects and therefore efforts have been focused on increasing the specificity of gene transfer to the targeted organ. Here, we describe a promoter containing six nuclear factor of activated T cells (NFAT) consensus sequences, which is as efficient as the cytomegalovirus (CMV) promoter to drive expression in vascular smooth muscle cells both in vitro and in vivo. In contrast to the CMV promoter it is activated in a Ca2+-dependent manner after endoplasmic reticulum depletion and allows the transgene expression only in proliferative/diseased cells. Overexpression of sarco/endoplasmic reticulum (SR/ER) Ca2+ ATPase 2a under the control of this NFAT promoter inhibits restenosis after angioplasty in rats. In conclusion, this promoter may be useful for gene therapy in vascular proliferative diseases and other diseases involving upregulation of the NFAT pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lipskaia L, Hulot JS, Lompre AM . Role of sarco/endoplasmic reticulum calcium content and calcium ATPase activity in the control of cell growth and proliferation. Pflugers Arch 2009; 457: 673–685.
Lompre AM, Hajjar RJ, Harding SE, Kranias EG, Lohse MJ, Marks AR . Ca2+ cycling and new therapeutic approaches for heart failure. Circulation 2010; 121: 822–830.
House SJ, Potier M, Bisaillon J, Singer HA, Trebak M . The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch 2008; 456: 769–785.
Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E et al. SUMO1-dependent modulation of SERCA2a in heart failure. Nature 2011; 477: 601–605.
Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 2011; 124: 304–313.
Aubart FC, Sassi Y, Coulombe A, Mougenot N, Vrignaud C, Leprince P et al. RNA interference targeting STIM1 suppresses vascular smooth muscle cell proliferation and neointima formation in the rat. Mol Ther 2009; 17: 455–462.
Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M et al. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res 2005; 97: 488–495.
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 1998; 93: 215–228.
Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF et al. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol 2002; 22: 7603–7613.
Wilkins BJ, Molkentin JD . Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol 2002; 541: 1–8.
Lipskaia L, Pourci ML, Deloménie C, Combettes L, Goudounèche D, Paul JL et al. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ Res 2003; 92: 1115–1122.
Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A . Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium 2007; 42: 145–156.
Lipskaia L, Lompre AM . Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell 2004; 96: 55–68.
Mancini M, Toker A . NFAT proteins: emerging roles in cancer progression. Nat Rev Cancer 2009; 9: 810–820.
Muller MR, Rao A . NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol 2010; 10: 645–656.
Szymanski P, Anwer K, Sullivan SM . Development and characterization of a synthetic promoter for selective expression in proliferating endothelial cells. J Gene Med 2006; 8: 514–523.
Pacak CA, Byrne BJ . AAV Vectors for Cardiac Gene Transfer: Experimental Tools and Clinical Opportunities. Mol Ther 2011; 19: 1582–1590.
Vallot O, Combettes L, Jourdon P, Inamo J, Marty I, Claret M et al. Intracellular Ca(2+) handling in vascular smooth muscle cells is affected by proliferation. Arterioscler Thromb Vasc Biol 2000; 20: 1225–1235.
Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 2004; 94: 110–118.
Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 2006; 116: 3114–3126.
Nakayama H, Otsu K, Yamaguchi O, Nishida K, Date MO, Hongo K et al. Cardiac-specific overexpression of a high Ca2+ affinity mutant of SERCA2a attenuates in vivo pressure overload cardiac hypertrophy. Faseb J 2003; 17: 61–63.
Bobe R, Hadri L, Lopez JJ, Sassi Y, Atassi F, Karakikes I et al. SERCA2a controls the mode of agonist-induced intracellular Ca(2+) signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol 2011; 50: 621–633.
Hulot JS, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y et al. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation 2011; 124: 796–805.
Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK et al. Orai1-mediated ICRAC is essential for neointima formation after vascular injury. Circ Res 2011; 109: 534–542.
del Monte F, Hajjar RJ, Harding SE . Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure. Circ Res 2001; 88: E66–E67.
Miyazaki M, Fujikawa Y, Takita C, Tsumura H . Tacrolimus and cyclosporine A inhibit human osteoclast formation via targeting the calcineurin-dependent NFAT pathway and an activation pathway for c-Jun or MITF in rheumatoid arthritis. Clin Rheumatol 2007; 26: 231–239.
Thomas TP, Patri AK, Myc A, Myaing MT, Ye JY, Norris TB et al. In vitro targeting of synthesized antibody-conjugated dendrimer nanoparticles. Biomacromolecules 2004; 5: 2269–2274.
Maguire AM, Simonelli F, Pierce EA, Pugh Jr EN, Mingozzi F, Bennicelli J et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008; 358: 2240–2248.
Inagaki K, Fuess S, Storm TA, Gibson GA, Mctiernan CF, Kay MA et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 2006; 14: 45–53.
Koerber JT, Jang JH, Schaffer DV . DNA shuffling of adeno-associated virus yields functionally diverse viral progeny. Mol Ther 2008; 16: 1703–1709.
White SJ, Nicklin SA, Büning H, Brosnan MJ, Leike K, Papadakis ED et al. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation 2004; 109: 513–519.
Su H, Arakawa-Hoyt J, Kan YW . Adeno-associated viral vector-mediated hypoxia response element-regulated gene expression in mouse ischemic heart model. Proc Natl Acad Sci USA 2002; 99: 9480–9485.
Su H, Huang Y, Barcena A, Arakawa-Hoyt J, Grossman W, Kan YW . Adeno-associated viral vector delivers cardiac-specific and hypoxia-inducible VEGF expression in ischemic mouse hearts. Proc Natl Acad Sci USA 2004; 101: 16280–16285.
Eggermont JA, Wuytack F, Verbist J, Casteels R . Expression of endoplasmic-reticulum Ca2(+)-pump isoforms and of phospholamban in pig smooth-muscle tissues. Biochem J 1990; 271: 649–653.
del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 1999; 100: 2308–2311.
Acknowledgements
This work was supported by Association Française contre les Myopathies (AFM). Dr Marchand and Dr Lompré were supported by the Leducq Foundation. Dr Lipskaia is supported by AHA SDG 0930116N. Dr Lompré benefits of a contract with Assistance publique-hôpitaux de Paris. Elise Merlet was supported by the French Ministry of research and education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Merlet, E., Lipskaia, L., Marchand, A. et al. A calcium-sensitive promoter construct for gene therapy. Gene Ther 20, 248–254 (2013). https://doi.org/10.1038/gt.2012.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.30
Keywords
This article is cited by
-
Inflammation-inducible promoters to overexpress immune inhibitory factors by MSCs
Stem Cell Research & Therapy (2023)
-
Efficient transduction of vascular smooth muscle cells with a translational AAV2.5 vector: a new perspective for in-stent restenosis gene therapy
Gene Therapy (2013)