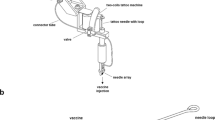
Abstract
The magnitude of the immune response to a DNA vaccine depends on three criteria—the optimized vector design, the use of a suitable adjuvant and the successful delivery and subsequent expression of the plasmid in the target tissue. In vivo electroporation (EP) has proved to be particularly effective in efficiently delivering DNA immunogens to the muscle and the skin, and indeed several devices have entered into human clinical trials. Here, we report on a novel concept of DNA delivery to the dermal tissue using a minimally invasive EP device, which is powered using low-voltage parameters. We show that this prototype device containing a novel 4 × 4-electrode array results in robust and reproducible transfection of dermal tissue and subsequent antigen expression at the injection site. Using DNA encoding for NP and M2e influenza antigens, we further show induction of potent cellular responses in a mouse model as measured by antigen-specific T-cell ELISpot assays. Importantly, 100% of the immunized animals were protected when challenged with VN/1203/04 (H5N1) strain of influenza. We have also extended our findings to a guinea-pig model and demonstrated induction of HI titers greater than 1:40 against a pandemic novel H1N1 virus showing proof of concept efficacy for DNA delivery with the prototype device in a broad spectrum of species and using multiple antigens. Finally, we were able to generate protective HI titers in macaques against the same novel H1N1 strain. Our results suggest that the minimally invasive dermal device may offer a safe, tolerable and efficient method to administer DNA vaccinations in a prophylactic setting, and thus potentially represents an important new option for improved DNA vaccine delivery in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Weiner DB . DNA vaccines: crossing a line in the sand introduction to special issue. Vaccine 2008; 26: 5073–5074.
Donnelly JJ, Ulmer JB, Shiver JW, Liu MA . DNA vaccines. Annu Rev Immunol 1997; 15: 617–648, review.
Andre S, Seed B, Eberle J, Schraut W, Bültmann A, Haas J . Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J Virol 1998; 72: 1497–1503.
Deml L, Bojak A, Steck S, Graf M, Wild J, Schirmbeck R et al. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J Virol 2001; 75: 10991–11001.
Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN . Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol 1997; 71: 4892–4903.
Muthumani K, Zhang D, Dayes NS, Hwang DS, Calarota SA, Choo AY et al. Novel engineered HIV-1 East African Clade-A gp160 plasmid construct induces strong humoral cell-mediated immune responses in vivo. Virology 2003; 314: 134–146.
Yang JS, Kim JJ, Hwang D, Choo AY, Dang K, Maguire H et al. Induction of potent Th1-type immune responses from a novel DNA vaccine for West Nile virus New York isolate (WNV-NY1999). J Infect Dis 2001; 184: 809–816.
Gao F, Weaver EA, Lu Z, Li Y, Liao HX, Ma B et al. Antigenicity immunogenicity of a synthetic human immunodeficiency virus type 1 group m consensus envelope glycoprotein. J Virol 2005; 79: 1154–1163.
Yan J, Yoon H, Kumar S, Ramanathan MP, Corbitt N, Kutzler M et al. Enhanced cellular immune responses elicited by an engineered HIV-1 subtype B consensus-based envelope DNA vaccine. Mol Ther 2007; 15: 411–421.
Toebak MJ, Gibbs S, Bruynzeel DP, Scheper RJ, Rustemeyer T . Dendritic cells: biology of the skin. Contact Dermatitis 2009; 60: 2–20, review.
Tobin DJ . Biochemistry of human skin—our brain on the outside. Chem Soc Rev 2006; 35: 52–67, review.
Martinon F, Kaldma K, Sikut R, Culina S, Romain G, Tuomela M et al. Persistent immune responses induced by a human immunodeficiency virus DNA vaccine delivered in association with electroporation in the skin of nonhuman primates. Hum Gene Ther 2009; 20: 1291–1307.
Song JM, Kim YC, Lipatov AS, Pearton M, Davis CT, Yoo DG et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B and T cell responses in mice. Clin Vaccine Immunol 2010; 17: 1381–1389.
Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR . Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine 1995; 13: 1427–1430.
Fuller DH, Murphey-Corb M, Clements J, Barnett S, Haynes JR . Induction of immunodeficiency virus-specific immune responses in rhesus monkeys following gene gun-mediated DNA vaccination. J Med Primatol 1996; 25: 236–241.
Bråve A, Ljungberg K, Boberg A, Rollman E, Isaguliants M, Lundgren B . et al. Multigene/multisubtype HIV-1 vaccine induces potent cellular humoral immune responses by needle-free intradermal delivery. Mol Ther 2005; 12: 1197–1205.
Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M et al. Increased DNA vaccine delivery immunogenicity by electroporation in vivo. J Immunol 2000; 164: 4635–4640.
Prud′homme GJ, Draghia-Akli R, Wang Q . Plasmid-based gene therapy of diabetes mellitus. Gene Ther 2007; 14: 553–564.
Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 2004; 22: 2489–2493.
Otten GR, Schaefer M, Doe B, Liu H, Megede JZ, Donnelly J et al. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine 2006; 24: 4503–4509.
Mathiesen I . Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther 1999; 6: 508–514.
Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB . Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 2008; 26: 440–448.
Rabussay DP, Nanda GS, Goldfarb PM . Enhancing the effectiveness of drug-based cancer therapy by electroporation (electropermeabilization). Technol Cancer Res Treat 2002; 1: 71–82.
Tjelle TE, Salte R, Mathiesen I, Kjeken R . A novel electroporation device for gene delivery in large animals and humans. Vaccine 2006; 24: 4667.
Draghia-Akli R, Khan AS, Brown PA, Pope MA, Wu L, Hirao L et al. Parameters for DNA vaccination using adaptive constant-current electroporation in mouse and pig models. Vaccine 2008; 26: 5230–5237.
Laddy DJ, Yan J, Khan AS, Andersen H, Cohn A, Greenhouse J et al. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J Virol 2009; 83: 4624–4630.
Laddy DJ, Yan J, Kutzler M, Kobasa D, Kobinger GP, Khan AS et al. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS One 2008; 3: e2517.
Gasparić M, Rubio I, Thönes N, Gissmann L, Müller M . Prophylactic DNA immunization against multiple papilloma virus types. Vaccine 2007; 25: 4540–4553.
Thönes N, Müller M . Oral immunization with different assembly forms of the HPV 16 major capsid protein L1 induces neutralizing antibodies and cytotoxic T-lymphocytes. Virology 2007; 369: 375–388.
Acknowledgements
We thank Ben Guiterrez, Melissa Pope, Maria Yang and Janess Mendoza for technical help with plasmid preparation, assays and animal care and procedures. This work was supported, in part, by US Army grant number W23RYX-8141-N604: #08023003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors KEB, XS, FL, JM, AK, SK and NYS are employees of Inovio. DBW has possible commercial interest associated with this work, which may include Inovio, VGX, VGXi, Wyeth, BMS, Medarex, Virxsys, Ichor, Merck, Althea and Aldevron.
Rights and permissions
About this article
Cite this article
Broderick, K., Shen, X., Soderholm, J. et al. Prototype development and preclinical immunogenicity analysis of a novel minimally invasive electroporation device. Gene Ther 18, 258–265 (2011). https://doi.org/10.1038/gt.2010.137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2010.137
Keywords
This article is cited by
-
Safe and efficient novel approach for non-invasive gene electrotransfer to skin
Scientific Reports (2018)
-
Adipose tissue: a new target for electroporation-enhanced DNA vaccines
Gene Therapy (2017)
-
Chemokine-adjuvanted electroporated DNA vaccine induces substantial protection from simian immunodeficiency virus vaginal challenge
Mucosal Immunology (2016)
-
DNA vaccination strategy targets epidermal dendritic cells, initiating their migration and induction of a host immune response
Molecular Therapy - Methods & Clinical Development (2014)