Abstract
Antiretroviral therapy (ART) effectively slows the progression of AIDS. However, drug resistance and/or toxicity can limit the utility of ART in many patients. In this study, we assessed whether a viral vector-based vaccine can be used as a therapeutic vaccine in simian immunodeficiency virus (SIV)-infected monkeys. The effect of vaccinating SIVmac239-infected rhesus monkeys with an SIV gag and gp120-expressing adenovirus (Ad) vector vaccine and a modified vaccinia Ankara (MVA) vaccine was explored while being treated with ART. Rhesus monkeys were intravenously infected with 10 and 1000 TCID50 (50% tissue culture infectious dose) of SIVmac239. Two months after SIV infection, the monkeys received a 4-month treatment with ART. Some of the monkeys were immunized with adenovirus-based vaccine and MVA-based vaccine with 2 months interval during ART. Viral load, CD4 count and SIV-specific immune responses were observed for 7 months after interruption of ART. The vaccinated animals had higher (i) CD4 counts, (ii) SIV-specific cell-mediated immune responses and (iii) anti-SIV-neutralizing antibody (Ab) titers than monkeys treated with ART alone. More importantly, the vaccination significantly reduced the SIV RNA load from animals infected with a low dose of SIV (10 TCID50). The anti-SIV cell-mediated and humoral responses induced by the vaccination was inversely correlated with a reduction in SIV viral load and positively correlated with an increase in CD4+ T cell counts. These results suggest that vaccination can improve antiviral cell-mediated and humoral immunity, which may contribute to controlling viral replication.
Similar content being viewed by others
Introduction
Antiretroviral therapy (ART) has successfully decreased the morbidity and mortality of patients infected with human immunodeficiency virus (HIV).1 ART therapy following acute simian immunodeficiency virus (SIV) or HIV infection suppresses viral replication, even after interruption of ART.2, 3 However, the virus can latently persist in resting CD4+ T cells, preventing eradication of the HIV infection with ART alone.4, 5, 6 Although long-term continuous treatment with ART can suppress viral replication, such therapy can lead to drug-related toxicity and the suppression of virus-specific immunity.7 Virus-specific immunity increases when ART is interrupted,8 but the magnitude of this response is rarely sufficient to control viral replication in chronically infected individuals.9 Moreover, interruption of ART is frequently associated with a rebound in viral titers,10, 11 and may encourage the emergence of drug-resistant strains.12
Strong CD4+ and CD8+ virus-specific T cell responses can control HIV replication.13, 14 Ongoing efforts are directed toward harnessing vaccines to induce such responses.15, 16, 17, 18, 19 Toward that end, we examined the efficacy of vaccinating during ART. SIV-infected rhesus macaques were primed with adenovirus (Ad) expressing SIV gag and gp120 and then boosted with modified vaccinia virus Ankara (MVA) also expressing those antigens while being treated with ART. Earlier studies in mice and non-human primates document the safety and immunogenicity of the Ad5/35 replication-defective human adenovirus20, 21, 22 and the strong immunogenicity of the attenuated MVA-based vaccine used in this study.23, 24, 25, 26, 27 Results from this study indicate that vaccination combined with short-term ART has a significant positive impact on CD4+ T cell count, SIV viral load and immune responses after ART is discontinued.
Results
Immunization regimen
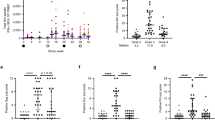
Initial experiments were performed in mice to identify an optimal immunization regimen for subsequent use in rhesus macaques. A single immunization with Ad5/35-HIV or MVA-HIV induced 5.2 and 3.2% HIV-specific tetramer+ CD8 cells 1 week later. Priming and boosting with the same vector induced stronger responses of 9.6 and 7.2%, respectively (both P<0.05) (Figure 1a). By comparison, priming with Ad5/35-HIV and boosting with MVA-HIV elicited the strongest response (14.5%, P<0.05). Similar results were obtained when the strength of the induced immune response was analyzed by an in vivo cytotoxic T lymphocyte (CTL) assay (both P<0.05) (Figure 1b). The Ad5/35-HIV prime/MVA-HIV booster also induced higher HIV-specific antibody than the homologous prime/booster with either Ad5/35-HIV or MVA-HIV (data not shown). On the basis of these results, an immunization regimen involving an Ad5/35 vector prime followed by a MVA vector boost was used in the therapeutic study of monkeys.
Cell-mediated immunity in mice. BALB/c mice (n=10 per group,) were primed and boosted intramuscularly with 109 v.p. (viral particles) of Ad5/35-HIV (human immunodeficiency virus) and/or 106 p.f.u. (plaque-forming units) of modified vaccinia Ankara (MVA)-HIV at 2-month intervals. An HIV-specific tetramer assay (a) and in vivo cytotoxic T lymphocyte (CTL) assay (b) were performed 1 week and 2 weeks after the last immunization, respectively. M2 represents the cells pulsed with HIV peptide, and M1 represents the cells without HIV peptide pulse.
SIV viral load
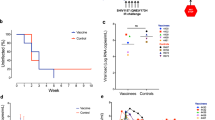
Monkeys were infected with 10 or 1000 TCID50 (50% tissue culture infectious dose) of SIVmac239 (Figure 2). SIV viral load peaked on day 10, and was significantly lower in the group challenged with a low dose of SIV (P<0.05, Figures 3a–e). In group 1 (infected, untreated), the viral load gradually increased with one monkey (1G0043) dying from opportunistic infection on day 233. In groups 2–4, ART reduced SIV replication (group 1 vs groups 2, 3 or 4, P<0.0001). There was no significant difference in viral load among groups 2–4 through days 60–180 (P>0.05). Interruption of ART resulted in the rapid rebound of SIV RNA in groups 2 and 3, but not group 4. Viral load was undetectable in three of the four monkeys in group 4 even after ART was discontinued, and viral titers were significantly lower in group 4 vs group 3 at all time points after day 293 (P<0.0001).
Monkey immunizing regimen. Monkeys (n=4–6 per group) were infected with simian immunodeficiency virus (SIV)mac239. Two months later, antiretroviral therapy (ART) was initiated (PMPA was administered at 30 mg kg−1 day−1 for 3 months and then at 20 mg kg−1 day−1 for the final month). Animals were primed with the Ad5/35-SIV vector and boosted with the MVA-SIV vector.
T cell count
CD4+ T cell number is an important index of SIV clinical stage and treatment efficacy. SIV infection rapidly reduced the number of CD4+ T cells in all groups (Figures 4a–e), with high dose infection destroying more CD4 cells than the low dose (group 3 vs group 4, P<0.0001). ART did not improve CD4+ T cell number during or after treatment (P>0.05, group 1 vs group 2). Vaccinated animals in group 3 experienced a significant increase in CD4+ count after ART through days 180–360 (group 2 vs group 3, P<0.05; group 1 vs group 3, P<0.05). These data suggest that vaccination may reduce the loss of CD4+ T cells following SIV infection.
CD8+ T cell number did not differ through 180 days post infection (P>0.05), although CD8 cell loss was greater in groups 1–3 than group 4 (P<0.0001) (data not shown). After ART was discontinued, CD8 count rose significantly in group 3 vs group 2 (P<0.0001). However, there was no significant difference between groups in the number of memory CD4 T cells after ART was discontinued (Figure 4f). The CD4 count significantly inversely correlated with SIV RNA load (R=−0.55±0.07) after day 202 of SIV infection, but not at earlier time points.
SIV-specific cell-mediated immunity
Cell-mediated immunity was monitored by interferon (IFN)γ ELISpot assay. SIV infection alone induced a poor IFNγ response (<150 p.f.u. (plaque-forming units) per million cells on an average, Figure 5a). ART alone increased the number of IFNγ-secreting T cells to <500 p.f.u. per million cells (Figure 5b). Vaccination significantly increased the number of SIV-specific immune cells both in groups 3 and 4 (P<0.05). This response declined after ART therapy in group 3, but was sustained in group 4 (Figures 5c and d). Interestingly, peak IFNγ-producing cell numbers were lower in group 4 than group 3 during ART (P<0.0001) (Figure 5e), but higher after ART (P<0.05). This ELIspot response was positively correlated with CD4+ T cell count (R=0.64±0.04) and inversely correlated with SIV RNA load (R=−0.59±0.07) after day 202 of SIV infection. These results suggest that vaccination elicits SIV-specific cell-mediated immunity, and that such an immunity may contribute to improving the CD4 T cell count and suppressing SIV replication.
Antigen (Ag)-specific interferon (IFN)γ-secreting cell numbers. Peripheral blood mononuclear cells (PBMCs) were stimulated using a pool of overlapping 15 aa from SIVmac239 gag and gp120. (a–d) Show IFNγ-secreting cells per million PBMCs for each animal per group (monkey 1G0043 died from AIDS on day 233), whereas (e) shows the average for all animals per group.
SIV-specific Ab
SIV-specific anti-Gag and anti-Env antibodies (Abs) were monitored by enzyme-linked immunosorbent assay. An anti-Gag Ab was first detected 3 weeks after SIV infection (Figures 6a–e). With group 1, SIV-specific Ab was increased 2 weeks after SIV infection (Figure 6a). On an average, vaccination significantly increased Ab titer (group 3 or 4 vs group 1 or 2, P<0.0001) during and after ART. A low dose of SIV infection elicited higher anti-Gag Ab than a higher dose of SIV infection, during (P<0.05) and after (P<0.0001) ART (Figure 6e). The titer of anti-SIV Gag Ab was positively correlated with the CD4 count (R=0.50±0.05), IFNγ ELIspot response (R=0.52±0.06) and was inversely correlated with the SIV viral load (R=−0.66±0.03) after day 202 of SIV infection.
Anti-Env Abs gradually increased in all groups of monkeys (Figures 7a–e). Group 4 generated higher Ab titers than group 3 from days 60 to 385 (P<0.0001). The titer of SIV env was positively correlated with CD4 count (R=0.44±0.03), ELIspot response (0.45±0.03) and was inversely correlated with the SIV viral load (R=−0.44±0.02). Importantly, the neutralizing Ab response of groups 3 and 4 significantly exceeded those in groups 1 and 2 (Figure 7f, P<0.05). These results suggest that vaccination improves the anti-SIV and neutralizing Ab response, and that humoral immunity correlates with cell-mediated responses and CD4+ T cell count after ART therapy plus vaccination.
Anti-SIV Env Ab titer and neutralizing Ab. SIV-specific Ab titers were examined at the indicated times. (a–d) show anti-SIV Env Ab titer for each animal per group (monkey 1G0043 died from AIDS on day 233), whereas (e) shows average titer for all animals per group. (f) Shows neutralizing antibody titer.
Discussion
This study explores the utility of combining a prime/boost vaccination strategy with ART to improve the host immune response and lower viral load in SIV-infected monkeys. Results indicate that vaccination markedly enhances SIV-specific cell-mediated and humoral responses, resulting in a higher CD4+ T cell count. It should be noted that vaccination controlled virus replication in three out of four monkeys infected with 10 TCID50 of SIV. By comparison, virus replication was only partially and temporally suppressed among vaccinated monkeys infected with 1000 TCID50 of SIV. Either the SIV viral load or the CD4 count was correlated with the induction of SIV-specific cell-mediated and humoral immune responses. These results suggest that vaccinating retrovirus-infected primates under the cover of ART may represent a promising approach to viral therapy.
Several studies on therapeutic vaccine combined with ART have been reported earlier. ART began in acute SIV infection28, 29 or chronic SIV infection.15, 16, 18, 19 DNA vaccine,29 poxvirus vector vaccine15, 16, 28 and virus-pulsed dendritic cells18, 19 have been used for vaccination. Some of the studies showed suppression of viral replication after interruption of ART. In this study, we used a prime/boost regimen with two strong viral vectors, adenovirus vector and MVA vector. Although SIV-specific immune responses significantly inversely correlated with SIV viral titer after interruption of ART, the viral replication was not controlled when the monkeys were infected with a high dose of SIV (Figure 3). Compared to other studies, the failure of controlling viral replication may be caused by differences in the dose of SIV infection, species of rhesus macaque, beginning time point of ART and its durability. Earlier study indicates that CD4+ central memory T cells rather than the viral load are correlated to AIDS progression.30 Activated memory CD4+ T cells in the gut-associated lymphoid tissue serve as an early target of HIV and a site of high viral replication.31 Severe depletion of CD4+ T cells occurs in the gut-associated lymphoid tissue rather than peripheral blood.32 We found that the control of viral replication, increased CD4 count and CTL activity in the monkeys infected with a low dose of SIV (Figures 3e and 6e) may result from low memory T cell depletion in primary SIV infection, although there are no differences in memory T cells in peripheral blood during chronic infection (Figure 5g).
Earlier studies indicate that CTL activity can limit HIV/SIV replication and reduce disease progression.13, 14 Initial studies involving mice showed that the combination of an Ad5/35 prime and MVA vector boost (both encoding HIV env gene) generated significantly higher CTL activity than prime/boost with a single viral vector (Figure 1). These results are consistent with those of Casimiro et al.,33 despite differences in the timing of the prime/boost. It should be noted that we observed that priming with Ad5/35 elicited neutralizing antivector antibodies that limited the subsequent immunogenicity of that vector if boosting was delayed by more than 4 weeks (data not shown).
SIV infection alone induced a weak SIV-specific CTL response (<150 IFNγ-secreting cells per million). ART modestly enhanced this response, but vaccination was needed to elicit robust CTL activity (Figure 5). A single prime/boost vaccination strategy induced CTL activity that persisted only 2 months after boost, indicating that effector CTL cells might be eliminated soon after vaccination. Interestingly, high CTL activity lasted longer in vaccinated animals infected with a lower dose of SIV (group 4 vs group 3, Figure 5). As increased CTL activity was inversely correlated with a reduction in SIV viral load, the persistence of this CTL response may contribute to the long-term suppression of SIV replication (Figure 3). These results were consistent with the clinical observation that HIV replication was suppressed only in patients with strong HIV-specific immunity, after interruption of ART.9
It was recently reported that a large-scale clinical study involving the use of an Ad5-vectored HIV vaccine was discontinued because vaccination did not reduce the incidence of HIV infection or reduce the HIV viral load after infection.34 That vaccine contained three Ad5 vectors expressing the HIV gag, pol and nef genes. There are several potential explanations for the failure of this vaccine to protect from HIV infection including (1) the HIV-specific immunity elicited by a single viral vector may be insufficient for inducing protection. We found that vaccination with multiple viral vectors induced a much higher CTL response than multi-vaccination with a single viral vector (Figure 1); (2) vaccine-induced immunity might be short lived. Our earlier study indicates that high HIV-specific CTL activity lasted for only 2 months after a single injection of an Ad-based vaccine.21 Multiple boosting may be necessary to achieve long-term protection; (3) memory T cells may not be induced effectively, as the Ad vector is a replication-deficient viral vector; (4) E1-deficient Ad5 vector was used. Presently, we used an Ad vector lacking both the E1 and E3 genes. Minimizing the number of viral genes in a vaccine vector may help strip the virus of its natural ability to evade the immune system against Ad vector. Thus, the promising results generated in this study may reflect the improved nature of the vaccine and/or improved schedule of the vaccination; (5) In the Merck study, the vaccine appeared to increase the rate of HIV infection in individuals with prior immunity against the Ad vector. Therefore, promising results may be available for our vaccine in HIV-infected individuals with low prior immunity against Ad vector and MVA vector.
HIV/SIV infection causes a biphasic destruction of CD4+ T cells. A massive loss of memory CD4 T cells mediated by direct viral infection occurs acutely,35, 36 followed by a slow but progressive loss of CD4+ T cells mediated by numerous chronic processes.37, 38 Those memory CD4+ T cells that survive the initial virus-mediated purge may subsequently expand, with several studies suggesting that prophylactic vaccination may help preserve and maintain these memory CD4 T cells.30, 39 In this study, memory CD4 T cells were detected in peripheral blood mononuclear cells 8 months after SIV infection. However, neither ART nor vaccination appeared to influence the size of the memory population (Figure 4f). These findings suggest that it may be very difficult for the memory CD4 T cell pool to recover after initial infection. In contrast, vaccination did trigger a significant increase in the number of naive CD4 (but not CD8) T cells present after ART was discontinued (Figure 4, data not shown). These findings suggest that vaccination may serve to activate CD4 T cells, thereby improving the CTL response. In this context, CD4 T cell count was inversely correlated with SIV viral load (Figures 3a–e and 5a–e). These findings confirm that ART rapidly controls SIV replication (Figures 3a–e). Vaccination did elicit both anti-Gag and anti-Env Abs. The magnitude of this Ab response paralleled CTL activity (Figures 6 and 7). Furthermore, serum-neutralizing titers were significantly higher in the vaccinated monkeys (groups 3, 4 vs 1, 2, Figure 7f). However, earlier studies show that neutralizing activity may be insufficient to protect T cells from SIV infection, as neutralizing Ab has difficulty in blocking CCR5-tropic viruses, such as SIVmac239.40
In the initial design of the experiment, we hope that ART can completely suppress SIV replication. Strong SIV-specific immunity induced by vaccination can control SIV replication after interruption of ART. This study used the reverse transcriptase inhibitor (R)-9-(2-phosphonylmethoxypropyl) adenine (PMPA) to treat SIV. PMPA was used at a dose of 20–30 mg kg−1 day−1 for 4 months. Consistent with clinical practice using monotherapy against HIV, even the highest dose of PMPA was only able to partially suppress SIV replication (Figures 3a–e). It should be noted that the administration of PMPA led to a significant loss of weight in most SIV-infected monkeys (data not shown). Thus, this monotherapeutic ART regimen often used in the SIV macaque infection model may be suboptimal. The viral load of three monkeys in group 4 was under the limit of detection (Figure 3d). The control of virus replication may be caused by a low dose of SIV infection and/or ART. However, ART did not completely suppress the viral replication during ART; moreover, viral load rebounded in all of the group 4 monkeys soon after the interruption of ART. We explain that the low viral load in the three monkeys of group 4, may have resulted from the strong cell-mediated immunity (Figure 5d). One of the group 4 monkeys (4G0275) with low CD4 count and high viral load may be the result of immune epitope(s) escape or novel MHC allele (Figures 3d and 4d).
The majority of published study involving therapeutic immunization has been conducted with Indian rhesus monkeys. In Indian rhesus monkeys there are certain MHC alleles associated with improved control of viral replication and improved prognosis, independent of treatment and/or immunization, notably Mamu-B*0841 and Mamu-B*17,42 and to a lesser extent, Mamu-A*01. It is likely that similar alleles associated with improved control of viral replication in the absence of treatment or vaccination also exist for Chinese rhesus monkeys. However, all of the monkeys used in this study were not selected with their MHC allele. The difference in MHC alleles may have a lesser effect on the results of the study.
In summary, this study explored a novel therapeutic regimen involving vaccination using a combination of virus vectors plus ART in a monkey model. Results indicate that this combination enhanced antigen-specific immune responses and CD4 T cell count and suppressed SIV replication once ART therapy was discontinued. These findings suggest that such a regimen is worthy of consideration for future clinical trials.
Materials and methods
Animals
Eight-week old BALB/c mice were used in the immunization regimen study, whereas 6-year-old male rhesus monkeys were used for the therapeutic study. The Chinese rhesus monkeys were maintained according to standard operating procedures established for the evaluation of human vaccines at the Animal Center, Chinese Medical Scientific Institute, Beijing, China. The study was permitted by the Animal Administer Community of Chinese Medical Scientific Institute and in accordance with requirements specified in the laboratory biosafety manual of the World Health Organization.
Viruses
An Ad5/35 expressing HIVIIIB gp160 (Ad5/35-HIV21) and MVA expressing HIVBH2 gp160 (MVA-HIV, kind gift of Dr Bernard Moss, National Institutes of Health, Rockville, MD, USA) were used. The SIVmac239 virus lot (named SIVman239/nef prepared in CEMx174 cells)43 used in challenge experiments was kindly provided by Dr Thomas Friedrich, Wisconsin National Primate Research Center, Madison, WI, USA. Two viral vectors were used in the monkey study as vaccines. One was an Ad5/35 vector21 expressing SIVmac239 gp120 and full length of Gag (Ad5/35-SIV). The other was an MVA vector expressing SIVmac239 gp120 and full length of Gag23, 24, 25, 26, 27 (MVA-SIV). The Ad5/35 virus was propagated in HEK293 cells and purified over CsCl as described elsewhere.44 The total concentration of virions in each preparation was calculated by the formula 1 OD260=1012 viral particles (v.p.) per ml. MVA virus was propagated in the BHK21 cell line and purified by one round of ultracentrifugation over 36% sucrose. The MVA virus was titrated in the BHK21 cell line to determine the number of p.f.u..
Immunization and ART
Monkeys of groups 1, 2 and 3 received 1000 TCID50 of SIVmac239 virus and monkeys of group 4 received 10 TCID50 of SIVmac239 by intravenous injection on day 0 (Figure 2). Monkeys of groups 2, 3 and 4 were subcutaneously given PMPA (Gilead Sciences Inc., Foster City, CA, USA) at a dose of 30 mg kg−1 day−1 from day 60 to day 150, and 20 mg kg−1 day−1 from day 151 to day 180. Monkeys of group 3 and group 4 were intramuscularly immunized with 1012 v.p. of Ad5/35-SIV vaccine and 109 p.f.u. of MVA-SIV vaccine on days 74 and 134, respectively.
Antibodies and flow cytometry
Antibodies were purchased from BD Pharmingen (San Diego, CA, USA). Anti-mouse CD8-FITC (Ly-2) Ab was used for mouse tetramer staining, and anti-IFNγ-FITC and anti-CD8a-PE (Ly-2) antibodies were used for intracellular cytokine staining. Anti-human CD3-PerCP, CD4-FITC and CD8-PE antibodies were used for staining monkey CD4 and CD8 cells. For the detection of memory CD4 T cells, CD3-Cy7APC, CD4-cascade blue, CD45RA-TRPE and CD95-APC were used; naive T cells were CD95-CD45RA+ and all other cells were memory T cells as described earlier.39
Tetramer assay and in vivo CTL assay
These assays were performed as described earlier.45 The H-2Dd/p18 tetramer (RGPGRAFVTI, synthesized by NIH Tetramer Core Facility, Atlanta, GA, USA) labeled with PE was used for tetramer assay. Briefly, 100 μl of heparin-preserved whole mouse blood was stained with 0.5 μg of FITC-conjugated anti-mouse CD8 Ab and 0.05 μg of tetramer reagent at room temperature for 30 min. The cells were then fixed with 100 μl of OptiLyse B-Lysing solution (Beckman Coulter, Marseille Cedex, France) at room temperature for 10 min. Erythrocytes were lysed by adding 1 ml of H2O and washed with phosphate buffered saline (PBS) twice.
For the in vivo CTL assay,45 target cells (naive splenocytes) were pulsed with 10 μg ml−1 of the HIV V3 peptide (RGPGRAFVTI) at 37 °C for 1 h. Peptide-pulsed cells were labeled with 0.5–5 μM of 5,6-carboxy-succinimidyl-fluorescein-ester, washed and enumerated. For the assay, 5 × 106 pulsed and 5 × 106 unpulsed cells were combined in a final volume of 200 μl of PBS and injected intravenously into vaccinated mice. Mice were killed 24 h after in vivo target cell incubation; lymphocytes were used for CTL analysis. CTL activity was defined by the following formula: % killing=(1−(unpulsed cells/peptide-pulsed cells from unimmunized control)/(unpulsed/peptide pulsed from immunized group)) × 100.
ELISpot analysis
Ninety-six well flat-bottom plates (Millipore, Tokyo, Japan) were coated overnight with 10 μg ml−1 of anti-human IFNγ Ab (B27; BD Pharmingen), washed and blocked with 5% fetal calf serum in PBS. Peripheral blood mononuclear cells were isolated by density gradient centrifugation from EDTA-preserved rhesus monkey blood. The unfractionated peripheral blood mononuclear cells were washed in RPMI-1640 supplemented with 10% fetal calf serum and seeded into the Ab coated wells at 2 × 105 cells per well. Cells were stimulated in vitro with 1 μg ml−1 of SIVmac239 gp120 and a Gag peptide pool (overlapping 15 amino acid peptides provided by the AIDS Research and Reference Reagent Program, NIH, Rockville, MD, USA), 5 μg ml−1 PHA-M (Sigma-Aldrich, Tokyo, Japan), or medium alone. Cells were cultured for 18 h at 37 °C in a 5% CO2 in an air incubator. The plates were developed with 2 μg ml−1 of biotinylated rabbit polyclonal anti-human IFNγ antiserum (Invitrogen, Carlsbad, CA, USA), washed, and incubated with streptavidin-alkaline phosphatase (Southern Biotechnology, Birmingham, AL, USA). After a final rinse, NBT/BCIP (Pierce, Rockford, IL, USA) was added for 10–15 min, and the plates were washed and air-dried. Developed wells were imaged and spot-forming cells were counted using the KS ELISPOT compact system (Carl Zeiss, Oberkochen, Germany). Spot-forming cells were defined as a large black spot with a fuzzy border.
Competitive PCR
Peripheral blood mononuclear cells were isolated by density gradient centrifugation from EDTA-preserved rhesus monkey blood. Total genomic RNA was extracted from 200 μl of plasma using TRIzol (Invitrogen, Carlsbad, CA, USA). Quantitative, competitive RT-PCR was performed using a QuantiTect SYBR Green RT-PCR kit (Qiagen, Hilden, Germany) with a sense primer sequence (GTAACTATGTCCACCTGCCATTA, binding to SIV genome no 1476-1498) and an antisense primer sequence (CAGCCTCCTCGTTTATGATGT, binding to SIV gag region).46
Enzyme-Linked ImmunoSorbent Assay
Enzyme-linked immunosorbent assay was performed as described earlier.21 To summarize, 96-well microtiter plates were coated with 200 ng ml−1 of SIVmac239 gag or gp120 peptide pool. The plate was incubated overnight at 4 °C and blocked with PBS 1%-bovine serum albumin for 2 h at room temperature. Diluted antisera were added and incubated for 2 h at 37 °C, and bound Ig was quantified using an affinity-purified horseradish peroxidase-labeled anti-human Ab (Sigma). Ab titer was measured by OD450 and compared to a standard high-tittered antiserum.
Neutralizing Ab
One hundred-fold diluted antisera were mixed with SIVmac239 virus (the virus was passaged once in CEMx174 cells from SIVmac239 stock) and incubated on ice for 30 min. The mixture (100 μl) was incubated with equal volume of CEMx174 cells (multiple of infection: 0.001) at 37 °C for 3 h. After incubation, the cells were washed three times with PBS and cultured in RPMI-1640 medium containing 10% fetal calf serum at 37 °C in a 5% CO2 incubator for 5 days. SIV p27 protein in the culture supernatant was detected using a SIV-1 p27 antigen ELISA kit (ZeptoMetrix Corp., Buffalo, NY, USA).
Data analysis
All values were expressed as means±s.e. Statistical analysis (Student's t-test) of the experimental data and controls was conducted with two-way factorial analysis of variance. Significance was defined at P<0.05 or P<0.0001 in the statistical analysis using all the time points in a certain durability of each group. R value was calculated with two parameters of each monkey at the same time point.
References
Palella Jr FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338: 853–860.
Rosenberg ES, Altfeld M, Poon SH, Phillips MN, Wilkes BM, Eldridge RL et al. Immune control of HIV-1 after early treatment of acute infection. Nature 2000; 407: 523–526.
Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med 1999; 340: 1683–1684.
Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA 1997; 94: 13193–13197.
Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278: 1295–1300.
Havlir DV, Bassett R, Levitan D, Gilbert P, Tebas P, Collier AC et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA 2001; 286: 171–179.
Casazza JP, Betts MR, Picker LJ, Koup RA . Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol 2001; 75: 6508–6516.
Ortiz GM, Wellons M, Brancato J, Vo HT, Zinn RL, Clarkson DE et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc Natl Acad Sci USA 2001; 98: 13288–13293.
Ortiz GM, Nixon DF, Trkola A, Binley J, Jin X, Bonhoeffer S et al. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Invest 1999; 104: R13–R18.
Davey Jr RT, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA 1999; 96: 15109–15114.
Garcia F, Plana M, Vidal C, Cruceta A, O'Brien WA, Pantaleo G et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. Aids 1999; 13: F79–F86.
Martinez-Picado J, Morales-Lopetegi K, Wrin T, Prado JG, Frost SD, Petropoulos CJ et al. Selection of drug-resistant HIV-1 mutants in response to repeated structured treatment interruptions. Aids 2002; 16: 895–899.
Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 1997; 278: 1447–1450.
Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 1999; 283: 857–860.
Tryniszewska E, Nacsa J, Lewis MG, Silvera P, Montefiori D, Venzon D et al. Vaccination of macaques with long-standing SIVmac251 infection lowers the viral set point after cessation of antiretroviral therapy. J Immunol 2002; 169: 5347–5357.
Hel Z, Venzon D, Poudyal M, Tsai WP, Giuliani L, Woodward R et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med 2000; 6: 1140–1146.
Harrer E, Bäuerle M, Ferstl B, Chaplin P, Petzold B, Mateo L et al. Therapeutic vaccination of HIV-1-infected patients on HAART with a recombinant HIV-1 nef-expressing MVA: safety, immunogenicity and influence on viral load during treatment interruption. Antivir Ther 2005; 10: 285–300.
Lu W, Wu X, Lu Y, Guo W, Andrieu JM . Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med 2003; 9: 27–32.
Lu W, Arraes LC, Ferreira WT, Andrieu JM . Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med 2004; 10: 1359–1365.
Havenga MJ, Lemckert AA, Ophorst OJ, van Meijer M, Germeraad WT, Grimbergen J et al. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J Virol 2002; 76: 4612–4620.
Xin KQ, Jounai N, Someya K, Honma K, Mizuguchi H, Naganawa S et al. Prime-boost vaccination with plasmid DNA and a chimeric adenovirus type 5 vector with type 35 fiber induces protective immunity against HIV. Gene Therapy 2005; 12: 1769–1777.
Someya K, Xin KQ, Ami Y, Izumi Y, Mizuguchi H, Ohta S et al. Chimeric adenovirus type 5/35 vector encoding SIV gag and HIV env genes affords protective immunity against the simian/human immunodeficiency virus in monkeys. Virology 2007; 367: 390–397.
Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 2001; 292: 69–74.
Barouch DH, Santra S, Kuroda MJ, Schmitz JE, Plishka R, Buckler-White A et al. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J Virol 2001; 75: 5151–5158.
Ramsburg E, Rose NF, Marx PA, Mefford M, Nixon DF, Moretto WJ et al. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J Virol 2004; 78: 3930–3940.
Mayr A, Stickl H, Müller HK, Danner K, Singer H . [The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's transl)]. Zentralbl Bakteriol [B] 1978; 167: 375–390.
Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004; 428: 182–185.
Uberla K, Rosenwirth B, Haaft P, Heeney J, Sutter G, Erfle V . Therapeutic immunization with Modified Vaccinia Virus Ankara (MVA) vaccines in SIV-infected rhesus monkeys undergoing antiretroviral therapy. J Med Primatol 2007; 36: 2–9.
Fuller DH, Rajakumar PA, Wu MS, McMahon CW, Shipley T, Fuller JT et al. DNA immunization in combination with effective antiretroviral drug therapy controls viral rebound and prevents simian AIDS after treatment is discontinued. Virology 2006; 348: 200–215.
Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 2006; 312: 1530–1533.
Nilsson J, Kinloch-de-Loes S, Granath A, Sönnerborg A, Goh LE, Andersson J . Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. Aids 2007; 21: 565–574.
Guadalupe M, Sankaran S, George MD, Reay E, Verhoeven D, Shacklett BL et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol 2006; 80: 8236–8247.
Casimiro DR, Bett AJ, Fu TM, Davies ME, Tang A, Wilson KA et al. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J Virol 2004; 78: 11434–11438.
Sekaly RP . The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med 2008; 205: 7–12.
Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M . Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005; 434: 1093–1097.
Song K, Rabin RL, Hill BJ, De Rosa SC, Perfetto SP, Zhang HH et al. Characterization of subsets of CD4+ memory T cells reveals early branched pathways of T cell differentiation in humans. Proc Natl Acad Sci USA 2005; 102: 7916–7921.
Picker LJ, Watkins DI . HIV pathogenesis: the first cut is the deepest. Nat Immunol 2005; 6: 430–432.
Douek DC, Picker LJ, Koup RA . T cell dynamics in HIV-1 infection. Annu Rev Immunol 2003; 21: 265–304.
Mattapallil JJ, Douek DC, Buckler-White A, Montefiori D, Letvin NL, Nabel GJ et al. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J Exp Med 2006; 203: 1533–1541.
Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D . Pathogenesis of macrophage tropic HIV-1. Curr HIV Res 2005; 3: 53–60.
Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol 2007; 81: 8827–8832.
Wojcechowskyj JA, Yant LJ, Wiseman RW, O'Connor SL, O'Connor DH . Control of simian immunodeficiency virus SIVmac239 is not predicted by inheritance of Mamu-B*17-containing haplotypes. J Virol 2007; 81: 406–410.
O'Connor DH, Mothe BR, Weinfurter JT, Fuenger S, Rehrauer WM, Jing P et al. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J Virol 2003; 77: 9029–9040.
Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002; 415: 331–335.
Xin KQ, Mizukami H, Urabe M, Toda Y, Shinoda K, Yoshida A et al. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J Virol 2006; 80: 11899–11910.
Someya K, Xin KQ, Matsuo K, Okuda K, Yamamoto N, Honda M . A consecutive priming-boosting vaccination of mice with simian immunodeficiency virus (SIV) gag/pol DNA and recombinant vaccinia virus strain DIs elicits effective anti-SIV immunity. J Virol 2004; 78: 9842–9853.
Acknowledgements
We thank NIH Tetramer Core Facility (Atlanta, GA, USA) for the tetramer; Gilead Sciences Inc. (Foster City, CA, USA) for (R)-9-(2-phosphonylmethoxypropyl) adenine (PMPA); Drs Chuan Qin, Hong Gao and Li-San Pan (Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences) for animal care and treatment. This work was partially supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan; a grant for the Strategic Research Project of Yokohama City University, Japan; a grant from the Research Foundation for Advanced Medical Research Center and a grant from the Japanese National Institute of Biomedical Innovation (No. 05-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimada, M., Wang, HB., Kondo, A. et al. Effect of therapeutic immunization using Ad5/35 and MVA vectors on SIV infection of rhesus monkeys undergoing antiretroviral therapy. Gene Ther 16, 218–228 (2009). https://doi.org/10.1038/gt.2008.152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.152
Keywords
This article is cited by
-
Biodistribution and immunity of adenovirus 5/35 and modified vaccinia Ankara vector vaccines against human immunodeficiency virus 1 clade C
Gene Therapy (2022)
-
Can immunotherapy be useful as a “functional cure” for infection with Human Immunodeficiency Virus-1?
Retrovirology (2012)
-
Partial protection against SIV challenge by vaccination of adenovirus and MVA vectors in rhesus monkeys
Gene Therapy (2010)