Abstract
Purpose
Fragile X premutation (PM) carriers may experience difficulties conceiving a child probably due to fragile X–associated diminished ovarian reserve (FXDOR). We investigated which subgroups of carriers with a PM are at higher risk of FXDOR, and whether the number of AGG interruptions within the repeat sequence further ameliorates the risk.
Methods
We compared markers of ovarian reserve, including anti-Müllerian hormone, antral follicle count, and number of oocytes retrieved between different subgroups of patients with a PM.
Results
We found that carriers with midrange repeats size (70–90 CGG) demonstrate significantly lower ovarian reserve. Additionally, the number of AGG interruptions directly correlated with parameters of ovarian reserve. Patients with longer uninterrupted CGG repeats post–AGG interruptions had the lowest ovarian reserve.
Conclusion
This study connects AGG interruptions and certain CGG repeat length to reduced ovarian reserve in carriers with a PM. A possible explanation for our findings is the proposed gonadotoxicity of the FMR1 transcripts. Reduction of AGG interruptions could increase the likelihood that secondary RNA structures in the FMR1 messenger RNA are formed, which could cause cell dysfunction within the ovaries. These findings may provide women with guidance regarding their fertility potential and accordingly assist with their family planning.
Similar content being viewed by others
Introduction
Fragile X syndrome (FXS) (OMIM #300624) is the most common cause of heritable intellectual disability and is the leading single-gene defect associated with autism, affecting approximately 1:4,000 males and 1:8,000 females.1 FXS is caused by a CGG repeat expansion in the 5′UTR of the fragile X mental retardation (FMR1) gene on the X chromosome.1 Besides FXS, expansion of CGG repeats within the FMR1 gene is also associated with other disorders: fragile X–associated primary ovarian insufficiency (FXPOI)2 and fragile X–associated tremor ataxia syndrome.3 Whereas FXS is caused by CGG repeat length of over 200 (full mutation (FM)) and FMR1 gene silencing, it has been proposed that FXPOI and fragile X–associated tremor ataxia syndrome are caused by toxic FMR1 messenger RNA (mRNA)4,5 and/or expression of an aberrant FMRpolyG protein in premutation (PM) patients with 55–200 repeats.6
FXPOI is characterized by amenorrhea (or oligomenorrhea) and menopausal follicle-stimulating hormone (FSH) levels (>40 mIU/ml) before the age of 40,7 affecting 13–20% of female carriers with a PM.8,9 Several studies have found a relatively high prevalence of PM in the general population: ~1 in 150–300 females and ~1 in 400–850 males.1 This suggests that all physicians are likely to see patients with this condition. In addition, patients with a PM may also present with sub/infertility associated with fragile X–associated diminished ovarian reserve (FXDOR). Unless caused by an abrupt, usually iatrogenic factor (such as oophorectomy, gonadotoxic chemotherapy, radiation, or ovarian surgery), POI would logically be preceded by diminished ovarian reserve (DOR). However, not all women with ovarian dysfunction and DOR develop POI. For that reason, we would assume that DOR is a much more common feature associated with FMR1 PM than FXPOI specifically.
Although women with a PM tend to have higher cycle day 3 serum FSH levels when compared with healthy, age-matched controls, so far there has been no stratification of the level of DOR among carriers with a PM based on the repeat length.8 It was, however, shown that carriers with a PM with <100 CGG repeats demonstrate a lower response to controlled ovarian hyperstimulation and decreased fertilization rates compared with those with >100 CGG repeats.10 Also using questionnaires it has been previously reported that some carriers with a PM with the midrange repeat size (80–100 repeats) demonstrate lower fertility.11 Today, in addition to day 3 FSH and surveys, there are several more accurate parameters of ovarian reserve in our armamentarium, such as antral follicle count (AFC) and anti-Müllerian hormone (AMH).12,13 So far no correlation has been found between the AFC and CGG repeat number.14 One study showed that carriers with a PM whose FMR1 gene contains more than 70 repeats demonstrate poorer ovarian reserve (reduced AMH level) compared with their counterparts with <70 CGG repeats. However, it was not reported whether patients with midrange repeat length demonstrated lower ovarian reserve.13 Another group found that having midsize and high CGG repeat (80–120 repeats) was associated with lower number of retrieved oocytes.15 Nevertheless, due to discrepancies between the repeat sizes as well as markers of ovarian reserve used in these different studies, further studies are needed to tease out the exact repeat length range that represents the highest risk for FXDOR and subfertility or infertility.
The presence of AGG interruptions within the CGG repeat sequence could indicate a better fertility outcome for patients carrying a PM. It is known that CGG repeats in the FMR1 mRNA form stable secondary structures such as hairpins.16 It was observed that women with a PM and at least one AGG interruption within the CGG repeat sequence are less likely to have an expansion of CGG repeat length into a FM and consequently are less likely to have a child with FXS in the next generation.17,18,19 This could potentially be explained by inhibition of the formation of secondary structures at the DNA level, which in turn would diminish the chance of DNA polymerase stalling and slippage,20,21,22 otherwise responsible for repeat expansions. It has also been shown that RNA containing the FMR1 CGG repeats is also able to form secondary hairpin structures in vitro.16 Secondary structures in the FMR1 mRNA have the ability to sequester cellular RNA-binding proteins, leading to the loss of their function. Disruptions of the formation of these hairpins in the FMR1 mRNA by AGG sequences could destabilize hairpin formation16 and thus alleviate the severity of PM-associated disorders. So far, a correlation between the number of AGG interruptions within the CGG repeat sequence and outcome of fragile X–associated PM diseases has not been evaluated.
Here, we studied whether the repeat size and AGG interruptions influence the ovarian reserve in women carrying a PM. We found that carriers with a PM of 70–90 CGG repeats demonstrated the lowest AMH levels and AFC when compared with those carriers with <70 or >90 repeats as well as unaffected controls. In addition, we found that having AGG interruptions within the repeat sequence was correlated with improved ovarian reserve. These results suggest that AGG interruptions in the CGG repeat sequence might indeed lower the propensity of formation of secondary structures in FMR1 mRNA, thus preventing its gonadotoxic effect, otherwise responsible for ovarian dysfunction in these patients. Furthermore, our results show that women with a PM in the midrange repeat length have the highest risk for DOR. These results shed light on the mechanisms responsible for reduced ovarian reserve in patients carrying a PM, and can contribute to genetic and clinical counseling of women who are at risk for fragile X–associated ovarian dysfunction.
Materials and methods
Patient inclusion criteria
All patients who were diagnosed with a fragile X PM (55–200 CGG repeats within FMR1 gene) between 2009 and 2015, either at our institution or referred to us after being diagnosed at an outside institution, were assessed for potential inclusion. At our institution, Next Step pan-ethnic carrier screening is utilized to screen for FMR1 PM or a FM, among other 280 genetic diseases. Since 2009, all incoming patients at the Center for Reproductive Medicine at Weill Cornell Medicine are routinely tested for CGG repeat length within the FMR1 gene and parameters of ovarian reserve (cycle day 2 FSH level, AMH level, and cycle day 2/3 ultrasound for AFC determination). Patients included in our study are patients seeking care for either infertility or for already known genetic abnormalities or both. Patients are treated using in vitro fertilization (IVF) to help to conceive a child and IVF with preimplantation genetic diagnosis to avoid propagation of genetic abnormalities to the offspring. All of the women in the study were still menstruating with normal levels of cycle day 2 FSH level.
Once a PM has been detected, the patient was referred to genetic counseling, during which the option of risk-reducing preimplantation genetic diagnosis is discussed, among other interventions. All the carriers with a PM who had at least one parameter of ovarian reserve (AFC or AMH) determined, and for whom the information about the number of CGG repeats was available, were included in the final analysis. Two patients had a PM on both alleles. As a control group, we used age- and body mass index–matched healthy women who were treated due to male factor (sub/infertility because of low sperm counts) at our facility during the study period (Supplementary Table 2 online). In 2013, we started testing for the number of AGG interruptions within the CGG repeat sequence for patients with <100 repeats23 to further stratify the risk of expansion into a FM in the next generation. This testing was performed at Asuragen (Austin, TX) by polymerase chain reaction. The primary outcomes included mean AMH serum level, mean AFC, and the mean number of oocytes retrieved. If a patient had multiple IVF cycles, the mean number of retrieved oocytes was used. Secondary outcomes included correlations between the number of CGG repeats and parameters of ovarian reserve, and correlations between the number of AGG interruptions and parameters of ovarian reserve.
Serum AMH levels were determined using Access2 ELISA kit (Beckman Coulter, Jersey City, NJ). A standard curve was generated in parallel to the assay and utilized to convert the absorbance values to ng/ml. The lower limit of sensitivity was 0.16 ng/ml. The coefficient of variation was <10% across the standard curve for both intra- and interassay variability. AFC represents the sum of antral follicles (5–10 mm) in both ovaries that was determined using transvaginal ultrasound on cycle day 2 of a menstrual cycle.
Clinical protocols
Protocols for controlled ovarian hyperstimulation, oocyte retrieval, in vitro fertilization, and embryo transfer were conducted according to the previously outlined practice.24 Briefly, the patients were either downregulated with the use of gonadotropin-releasing hormone agonist (Lupron, Abbott Pharmaceuticals, Worcester, MA, USA) followed by stimulation with gonadotropins (Follistim, Merck, Whitehouse Station, NJ, USA; Gonal-F, EMD-Serono, Rockland, MA, USA; and/or Menopur, Ferring, Parsippany, NJ, USA) or were treated with gonadotropins until criteria for pituitary suppression with a gonadotropin-releasing hormone antagonist (0.25 mg Ganirelix acetate, Organon) were met.25 Human chorionic gonadotropin (Pregnyl, Merck) or a combination of human chorionic gonadotropin and gonadotropin-releasing hormone agonist was used as the ovulatory trigger when the two lead follicles reached a mean diameter >17 mm. Ultrasound-guided transvaginal oocyte retrieval was performed after 35–37 hours following the ovulatory trigger based on our standard practice.24
Statistical analysis
STATA Statistical Software version 11 (StataCorp LP, College Station, TX) was utilized for data analysis. Continuous variables were analyzed using Student’s t-test as the data was normally distributed. Categorical variables were analyzed by chi-square and Fisher’s exact test. A P < 0.05 was considered statistically significant for all tests indicated. Multiple linear regression was used to examine the relationship between two independent continuous variables: (i) the 3′ uninterrupted CGG repeats after determination of AGG interruption location within the affected allele and parameters of ovarian reserve (AMH and AFC), and (ii) the number of AGG interruptions within the affected allele and the parameters of ovarian reserve while controlling for confounder (patients’ age) and predictor variable (repeat size length). Because age is inversely linearly correlated to log odds of the outcome (parameters of ovarian reserve (AMH and AFC)), age was included in the linear model as a continuous variable to control for age in this model. To calculate the multivariate linear regression we used STATA Statistical Software version 11. We used then the R 2 (the coefficient of multiple determination for multiple regression) as statistical measure of how close the data are to the fitted regression line, which was also calculated with STATA. The Institutional Review Board of Weill Cornell Medicine approved the current study.
Results
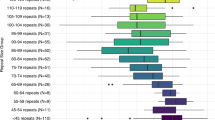
Carriers with a PM and a midrange number of CGG repeats have been found to have a higher risk of FXPOI.26 We were interested to test whether women with a PM and the midsize repeat length are at a higher risk for FXDOR than women with a PM with lower and higher repeat number. A total of 96 patients with a PM and 86 healthy controls were included in the final analysis (Table 1). Carriers with a PM were further divided into 2 groups based on the number of CGG repeats: 24 patients with 70–90 CGG repeats constituted the “midrange group,” while 72 of those with lower or higher number of CGG repeats were included in the “<70 & >90 group.” All groups, women with PM and controls, were matched for age and body mass index (Table 1). To test for ovarian reserve we compared the AMH level, AFC, and number of retrieved oocytes between those groups. We found that the AMH levels were decreased in women carrying a PM in comparison with the control group. In particular, the ovarian reserve parameters of ovarian reserve were significantly lower for midrange carriers with a PM in comparison with carriers with a PM with lower or higher repeat number (Table 1 and Figure 1). Midrange carriers with a PM demonstrated significantly lower AFC (Figure 1a) as well as mean serum AMH levels (Figure 1b) in comparison with patients with a PM with <70 and >90 CGG repeats. Furthermore, 62 of 96 patients with a PM underwent controlled ovarian hyperstimulation and oocyte retrieval, and again, the midrange group demonstrated significantly lower number of oocytes retrieved when compared with carriers with a PM with lower or higher repeat number (Figure 1c). These results show that carriers with a PM and 70 to 90 CGG repeats have the lowest ovarian reserve and the highest risk for FXDOR.
Carriers with a PM in the midrange group have (a) lower antral follicle count (AFC) and (b) lower anti-Müllerian hormone (AMH) level when compared with carriers with a PM with a lower or higher repeat number (56–70 or >90 CGG repeats). (c) In addition, fewer oocytes were retrieved from these patients. The number of patients is indicated in the diagram.
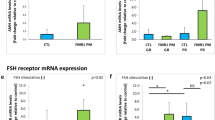
AMH is produced by the granulosa cells of growing ovarian follicles. Decreased AMH levels could be indicative of granulosa cell dysfunction and/or an overall decreased number of growing follicles. It has been suggested that the increased FMR1 mRNA in granulosa cells of patients with a PM could result in cellular gonadotoxicity by the formation of secondary RNA structures. These secondary structures could sequester cellular proteins, thus contributing to loss of their function in the cells15 and leading to cellular toxicity resulting in ovarian dysfunction. We hypothesize that AGG interruptions could prevent the formation of these secondary structures in the FMR1 mRNA, and cellular toxicity as well as ovarian dysfunction. To test this hypothesis, we sought to determine if AGG interruptions within the CGG repeat sequence correlates with ovarian reserve as reflected by the AMH level, AFC, and the number of oocytes retrieved. Starting in 2013 a total of 32 patients with a PM and fewer than 100 CGG repeats were tested for AGG interruptions in our center. The purpose of the testing was the emerging relevance of these interruptions for CGG repeat expansion to the FM range in the next generation, when the women are PM carriers17,18,19 (Table 2). When analyzing all carriers with a PM of age 35 or younger (19 patients), we found that the AMH of those with 2 AGG interruptions within the CGG repeat sequence was higher than those carriers with none or 1 AGG interruption (Figure 2a). Similar results were obtained for all carriers with a PM at all ages (32 patients, Supplementary Figure 2). Furthermore, using multiple linear regression analysis to control for age and repeat length, we observed in carriers with a PM and of all ages, whose AGG number has been determined (n = 32), a correlation between the number of AGG interruptions and AFC, and a trend in the correlation between the number of AGG interruptions and AMH when controlled for age and the number of CGG repeats (Supplementary Table 1). Although the number of patients with a PM and 0 AGG interruptions was small (only 3 patients) we detected a considerably higher ovarian reserve in patients with a PM and two AGG interruptions in contrast to one or no AGG interruptions.
(a) Three patients with a premutation (PM) with no AGG interruptions, 10 patients with a PM with one AGG interruption, and 6 patients with a PM with two AGG interruptions were in the study section. The anti-Müllerian hormone (AMH) levels were increased with the number of AGG interruptions in patients with a PM and age of 35 and younger. (b) AMH level and (c) the number of retrieved oocytes decreased with the length of longest remaining uninterrupted CGG repeat in patients with a PM, indicating that women with AGG interruptions have a higher ovarian reserve in comparison with carriers without AGG interruptions in the CGG repeat sequence (see Figure 3).
Interestingly, we also found that the AMH levels and AFC tended to be lower in those carriers with a PM with longer 3′ uninterrupted CGG-repeat fragments post–AGG interruptions (Figure 2b, Supplementary Figure 1). Accordingly, the number of retrieved oocytes was also lower in these patients, who underwent controlled ovarian hyperstimulation and oocyte retrieval (Figure 2c). Consistent with these results we found that while controlling for age, there was an inverse correlation between the 3′ uninterrupted CGG-fragment length post–AGG interruptions and AMH, and AFC (Supplementary Table 1). These results indicate that the number of the AGG interruptions within the CGG repeat sequence and the remaining repeat length after AGG interruptions (3′ uninterrupted CGG repeat-fragment) have an impact on the ovarian reserve in women with a PM.
Discussion
We were interested in investigating which PM repeat size, and whether the absence of AGG interruptions within the repeat sequence, ameliorates the risk of FXDOR. Our results demonstrate for the first time that AGG interruptions in the CGG repeat sequence have an effect on the fragile X–associated ovarian dysfunction in patients carrying a PM. It has been previously shown that the expansion to FM is reduced by AGG interruptions within the premutated allele.17,19 AGG interruptions are generally at the 5′ end of the CGG repeat sequence27,28,29 and it was suggested that these interruptions stabilize the repeats1 probably by preventing the formation of secondary repeat DNA structures within the cell. AGG interruptions alter and destabilize the structures formed by the repeats in vitro.30 However, secondary repeat structures could also form within the FMR1 RNA, as shown in vitro.16 These secondary RNA structures could interfere with normal cellular processes, further leading to ovarian dysfunction. AGG interruptions could decrease the chance or even completely inhibit the formation of these noncanonical structures in the FMR1 RNA, thus ensuring normal cellular and tissue functioning. Indeed, we found that reduced number of AGG interruptions, from 2 to 1 or none, was associated with increased risk of DOR in patients carrying a PM. In addition, women with a PM and AGG interruptions at the 5′ end of the CGG repeat leaving a longer 3′ uninterrupted CGG repeat fragment have a lower ovarian reserve. It seems that the repeat structure (AGG interruptions and 3′ uninterrupted repeat length) has an effect on the ovarian reserve regardless of age (Figure 2). Nevertheless, because our patient group was small (32 patients) it will be interesting to see whether this will be the case in a larger study group.
The PM is associated with increased FMR1 gene transcription but decreased translation, resulting in low to regular levels of the fragile X mental retardation protein (FMRP).31,32 Although one study showed that AGG interruptions seem not to influence translation efficiency of FMRP33 they might have an effect on the expression of a homopolymeric polyglycine-containing peptide (FMRpolyG). Repeat-associated non-UTG–initiated translation was suggested to lead to expression of FMRpolyG proteins in the brain of fragile X–associated tremor ataxia syndrome patients, which could explain certain symptoms of the PM pathogenesis.6 Recently, FMRpolyG proteins were also found in ubiquitin-positive inclusions in ovarian stromal cells of a women with a PM.34 While the mechanisms triggering repeat-associated non-UTG translation are unknown, it has been proposed that the scanning 43S ribosomal preinitiation complex stalls at the CGG repeats, allowing for usage of an alternated initiation codon, which leads to translation of FMRpolyG proteins.6 AGG interruptions could prevent stalling of the translation machinery at the repeats and lead to lower, or an absence of, FMRpolyG protein production. This could avoid cell toxicity in ovarian tissues and ovarian dysfunction.
It has been previously shown that carriers with a PM and midrange number of CGG repeats are at higher risk of developing FXPOI35 (reviewed in 26). It was also reported that carriers with a PM might suffer from sub- or infertility, even without developing FXPOI later in life.11,15 Although DOR precedes FXPOI, additional factors such as lifestyle, genetic background, and skewed X chromosome inactivation were suggested to contribute to the development of FXPOI in female FMR1 carriers.26 To determine the ovarian reserve in patients with a PM we analyzed three different parameters of ovarian reserve: AMH level, AFC, and the number of retrieved oocytes. Our results are consistent with previously published data, showing that carriers with a PM and midrange repeat size (70–90) have a significantly lower ovarian reserve in comparison with carriers with a PM and lower or higher CGG repeat number, as well as age-matched controls. We also compared the ovarian reserve between women with a PM and 80–100 CGG repeats with women with lower and higher CGG repeat numbers and did not find a significant difference between those groups. Skewed X-chromosome inactivation of the expanded allele could explain the decreased incidence of FXDOR and FXPOI in patients with a PM and more than 100 CGG repeats.36 Additionally, it could also explain why female carriers with a PM have a lower risk of developing fragile X–associated tremor ataxia syndrome in contrast to male carriers. However, it has been also reported that either no evidence or only in limited number of patients with a PM skewed X-chromosome inactivation of the normal length repeat might be a risk factor for the development of the PM pathology.37,38,39,40 Nevertheless, our results suggest that similar to FXPOI, carriers with a PM and midrange CGG repeats are also at a higher risk of developing FXDOR.
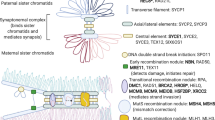
An increased FMR1 mRNA level was detected in ovarian granulosa cells of female carriers with a PM.15 Since the CGG repeats in FMR1 mRNA are able to form unusually secondary structures in vitro,16 we propose a model where disruption of the formation of these structures by AGG interruptions could result in lower probability of the formation of secondary repeat structure in the FMR1 RNA and decreased sequestering of RNA-binding proteins (Figure 3), which could inhibit cellular processes in the ovaries of women carrying a PM. In support of this model, our results show that women with a PM and AGG interruptions have higher ovarian reserve. To summarize, this study reveals that analysis of the CGG repeat length and AGG interruptions identifies women with a greater risk for FXDOR and infertility among carriers with a PM. However, because our sample size was limited, further studies with larger sample size would be desirable. Our results illuminate the importance of testing women for CGG repeat length along with AGG interruptions, especially in women of reproductive age who consider childbearing. If diagnosed early, women with a PM and midrange repeats and one or no AGG interruption could make an informed decision about their fertility and try to conceive earlier in life, with or without IVF, or peruse options of fertility preservation.
Uninterrupted CGG repeats (yellow) could form secondary structures within ovarian cells such as granulosa cells. These RNA structures could cause binding and sequestering of RNA-binding proteins (red balls), whose function would be lost in the cells causing cell toxicity. AGG interruptions (purple) in the repeat sequence could prevent the formation of these secondary repeat structures, and thus lower the sequestering of proteins in the cell. This in turn could result in less accumulation of proteins in the cells, and could prevent cell dysfunction in the ovary in women carrying a PM. mRNA, messenger RNA.
References
Nelson DL, Orr HT, Warren ST. The unstable repeats—three evolving faces of neurological disease. Neuron 2013;77:825–843.
Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet 2000;97:189–194.
Hagerman RJ, Leehey M, Heinrichs W et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001;57:127–130.
Hagerman RJ, Hagerman P. Fragile X-associated tremor/ataxia syndrome—features, mechanisms and management. Nat Rev Neurol 2016;12:403–412.
Mila M, Alvarez-Mora MI, Madrigal I, Rodriguez-Revenga L. Fragile X syndrome: an overview and update of the FMR1 gene. Clin Genet, e-pub ahead of print 15 June 2017.
Todd PK, Oh SY, Krans A et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 2013;78:440–455.
Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med 2009;360:606–614.
Sullivan AK, Marcus M, Epstein MP et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod 2004;20:402–412.
Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet 1999;83:322–325.
Bibi G, Malcov M, Yuval Y et al. The effect of CGG repeat number on ovarian response among fragile X premutation carriers undergoing preimplantation genetic diagnosis. Fertil Steril 2009;94:869–874.
Allen EG, Sullivan AK, Marcus M et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod 2007;22:2142–2152.
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006;12:685–718.
Rohr J, Allen EG, Charen K et al. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod 2008;23:1220–1225.
Maslow BS, Davis S, Engmann L, Nulsen JC, Benadiva CA. Correlation of normal-range FMR1 repeat length or genotypes and reproductive parameters. J Assist Reprod Genet 2016;33:1149–1155.
Elizur SE, Lebovitz O, Derech-Haim S et al. Elevated levels of FMR1 mRNA in granulosa cells are associated with low ovarian reserve in FMR1 premutation carriers. PLoS One 2014;9:e105121.
Napierala M, Michalowski D, de Mezer M, Krzyzosiak WJ. Facile FMR1 mRNA structure regulation by interruptions in CGG repeats. Nucleic Acids Res 2005;33:451–463.
Nolin SL, Glicksman A, Ersalesi N et al. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet Med 2014;17:358–364.
Eichler EE, Holden JJ, Popovich BW et al. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet 1994;8:88–94.
Yrigollen CM, Durbin-Johnson B, Gane L et al. AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet Med 2012;14:729–736.
Gerhardt J, Tomishima MJ, Zaninovic N et al. The DNA replication program is altered at the FMR1 locus in fragile X embryonic stem cells. Mol Cell 2013;53:19–31.
Mirkin SM. Expandable DNA repeats and human disease. Nature 2007;447:932–940.
Gerhardt J, Bhalla AD, Butler JS et al. Stalled DNA replication forks at the endogenous GAA repeats drive repeat expansion in Friedreich’s ataxia cells. Cell Rep 2016;16:1218–1227.
Nolin SL, Sah S, Glicksman A et al. Fragile X AGG analysis provides new risk predictions for 45-69 repeat alleles. Am J Med Genet A 2013;161A:771–778.
Davis OK, Rosenwaks Z. Superovulation strategies for assisted reproductive technologies. Semin Reprod Med 2001;19:207–212.
Reichman DE, Chung P, Meyer L, Greenwood E, Davis O, Rosenwaks Z. Consecutive gonadotropin-releasing hormone-antagonist in vitro fertilization cycles: does the elapsed time interval between successive treatments affect outcomes? Fertil Steril 2013;99:1277–1282.
Sherman SL, Curnow EC, Easley CA et al. Use of model systems to understand the etiology of fragile X-associated primary ovarian insufficiency (FXPOI). J Neurodev Disord 2014;6:26.
Kunst CB, Leeflang EP, Iber JC, Arnheim N, Warren ST. The effect of FMR1 CGG repeat interruptions on mutation frequency as measured by sperm typing. J Med Genet 1997;34:627–631.
Kunst CB, Warren ST. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell 1994;77:853–861.
Kunst CB, Zerylnick C, Karickhoff L et al. FMR1 in global populations. Am J Hum Genet 1996;58:513–522.
Jarem DA, Huckaby LV, Delaney S. AGG interruptions in (CGG)(n) DNA repeat tracts modulate the structure and thermodynamics of non-B conformations in vitro. Biochemistr. 2010;49:6826–6837.
Hagerman RJ, Hagerman PJ. The fragile X premutation: into the phenotypic fold. Curr Opin Genet Dev 2002;12:278–283.
Tassone F, Hagerman RJ, Taylor AK et al. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet 2000;91:144–152.
Ludwig AL, Raske C, Tassone F, Garcia-Arocena D, Hershey JW, Hagerman PJ. Translation of the FMR1 mRNA is not influenced by AGG interruptions. Nucleic Acids Res 2009;37:6896–6904.
Buijsen RA, Visser JA, Kramer P et al. Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency. Hum Reprod 2015;31:158–168.
Spath MA, Feuth TB, Smits AP et al. Predictors and risk model development for menopausal age in fragile X premutation carriers. Genet Med. 2011;13:643–650.
García-Alegría E, Ibáñez B, Mínguez M et al. Analysis of FMR1 gene expression in female premutation carriers using robust segmented linear regression models. RNA 2007;13:756–762.
Alvarez-Mora MI, Rodriguez-Revenga L, Feliu A, Badenas C, Madrigal I, Mila M. Skewed X inactivation in women carrying the FMR1 premutation and its relation with fragile-X-associated tremor/ataxia syndrome. Neurodegener Dis 2016;16:290–292.
Rodriguez-Revenga L, Madrigal I, Badenas C, Xuncla M, Jimenez L, Mila M. Premature ovarian failure and fragile X female premutation carriers: no evidence for a skewed X-chromosome inactivation pattern. Menopause 2009;16:944–949.
Murray A, Ennis S, MacSwiney F, Webb J, Morton NE. Reproductive and menstrual history of females with fragile X expansions. Eur J Hum Genet 2000;8:247–252.
Bione S, Benedetti S, Goegan M et al. Skewed X-chromosome inactivation is not associated with premature ovarian failure in a large cohort of Italian patients. Am J Med Genet A 2006;140:1349–1351.
Acknowledgments
This work was supported by a Starr Foundation and Perelman research recruitment gift. We thank Omar Alexander Man for the illustration.
Author information
Authors and Affiliations
Contributions
JG conceived and designed the study. JL received the data and performed the statistical analysis. KX, CC, DL, JS and NP obtained the data. JG, JL and LM analyzed and discussed the results. JL, JG and LM wrote the manuscript. LM designed Figure 3. JG, JL, LM and ZR edited the manuscript.
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lekovich, J., Man, L., Xu, K. et al. CGG repeat length and AGG interruptions as indicators of fragile X–associated diminished ovarian reserve. Genet Med 20, 957–964 (2018). https://doi.org/10.1038/gim.2017.220
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2017.220
Keywords
This article is cited by
-
Genetic determination of the ovarian reserve: a literature review
Journal of Ovarian Research (2021)
-
Does the presence of AGG interruptions within the CGG repeat tract have a protective effect on the fertility phenotype of female FMR1 premutation carriers?
Journal of Assisted Reproduction and Genetics (2020)
-
Are ovarian response and pregnancy rates similar in selected FMR1 premutated and mutated patients undergoing preimplantation genetic testing?
Journal of Assisted Reproduction and Genetics (2020)
-
Defining the role of FMR1 gene in unexplained recurrent spontaneous abortion
Journal of Assisted Reproduction and Genetics (2019)