Abstract
Purpose:
Familial hypercholesterolemia (FH) is one of the most common monogenic disorders, and the high concentrations of low-density lipoprotein (LDL) cholesterol presented since birth confers on these patients an increased cardiovascular risk. More than 1,600 alterations have been described in the LDL receptor gene (LDLR), but a large number need to be validated as mutations causing disease to establish a diagnosis of FH. This study aims to characterize, both at the phenotypic and genotypic levels, families with a clinical diagnosis of FH and present evidence for the importance of the integration of clinical, molecular, and functional data for the correct diagnosis of patients with FH.
Methods:
A detailed analysis of the phenotype and genotype presented by 55 families with 13 different alterations in the LDLR was conducted. For eight of these, an extensive functional characterization was performed by flow cytometry, confocal microscopy, and reverse transcriptase polymerase chain reaction.
Results:
Carriers of neutral alterations presented a significantly lower incidence of premature cardiovascular disease, lower levels of atherogenic lipoproteins and a large number of these individuals had LDL-cholesterol values below the 75th percentile. presented a significantly lower incidence of premature cardiovascular disease, lower levels of atherogenic lipoproteins and a large number of these individuals had LDL-cholesterol values below the 75th percentile However, the functional study was essential to determine the pathogenicity of variants.
Conclusion:
The data collected illustrate the importance of this integrated analysis for the correct assessment of patients with FH who can otherwise be misdiagnosed.
Genet Med 17 12, 980–988.
Similar content being viewed by others
Introduction
Familial hypercholesterolemia (FH) is one of the most common and well-known monogenic disorders; mutations in the low-density lipoprotein (LDL) receptor gene (LDLR) are the underlying cause in more than 90% of cases.1,2,3 Patients with FH have an increased cardiovascular risk as a result of the high concentrations of LDL cholesterol (LDL-C) present from birth.4 The World Health Organization recommends large-scale screening to identify patients who can most benefit from the early implementation of lipid-lowering treatment. The early identification and treatment of these patients can reduce or even eradicate their elevated cardiovascular risk if LDL-C concentrations are reduced for their lifetime.5 Clinical identification is possible, but in most cases it is not sufficient to identify these patients6; therefore, recent dyslipidemia guidelines recommend DNA testing whenever possible.7 In the 30 years since mutations in LDLR were identified as the prime defect in patients with FH, the laboratory techniques to identify these mutations have improved greatly. New variants are found every day in different populations,8,9,10 but the functional effect of these variants is usually not assessed, which can lead to misdiagnosis. With the novel sequencing technologies being applied for genetic diagnosis of FH, it is expected that an increasing number of variants of unknown clinical significance will be found in LDLR. Therefore, understanding whether theses alterations disturb the function of the protein becomes important. In fact, the last update of the FH database8 recognizes that, based on in silico analysis, only about 80% of all reported alterations are pathogenic, and more than half of the missense mutations reported so far do not have functional studies. Cosegregation studies have been very useful in assessing variant pathogenicity11,12,13; however, all these data are not usually analyzed as a whole when considering the genetic diagnosis of FH.
A detailed description and analysis of the phenotype and genotype presented by 55 families with a clinical diagnosis of FH carrying five functional and eight neutral alterations are discussed here. For this, an extensive functional characterization was performed for eight of these alterations without functional studies. An integrated discussion of clinical, molecular, and functional data is presented, highlighting the importance of this analysis for the correct assessment of patients with FH.
Materials and Methods
Study population
Data on phenotype/genotype of 135 participants of the Portuguese FH Study are presented. Written informed consent was obtained from all participants before their inclusion in the study. The study protocol and database were previously approved by the National Institute of Health Ethical Committee and National Data Protection Commitee.
Lipid profile
Fasting blood samples were collected from individuals at the time of their inclusion in the study. Total cholesterol, direct LDL-C, high-density lipoprotein cholesterol (HDL), triglycerides, apolipoprotein A1 (apoA1), apolipoprotein B (apoB), and lipoprotein(a) were determined for all individuals using Cobas Integra 400 plus (Roche, Basel, Switzerland) and enzymatic colorimetric and immunoturbidimetric methods.
For all individuals, LDL-C percentiles were calculated by age and sex, according to the reference values for the Spanish population,14 because of the absence of percentile distributions for fasting serum lipids in the Portuguese population. In some individuals there were only LDL-C values while receiving medication; in that case, those values were corrected for the type of medication. For statins, the reduction factor used was 30% (LDL-C under treatment ×1.3); for the combination of a statin and ezetimibe, the factor used was 50% (LDL-C under treatment ×1.5).15,16
Segregation analysis
The cosegregation of the alterations with the hypercholesterolemia in each family was evaluated and expressed as the number of “alteration carriers/total affected” or “alteration carriers/total nonaffected.” “Total affected” referred to all relatives with hypercholesterolemia (>75th percentile, adjusted for sex and age) and “total nonaffected” was related to all relatives with normal lipid values (<75th percentile, adjusted for sex and age).
Molecular analysis
A genetic diagnosis of FH was made by the molecular study of the APOB (fragments of exons 26 and 29), LDLR (including the study of large rearrangements), and PCSK9 genes, as reported previously.11
In silico analysis
The predicted effects of LDLR nonsynonymous missense alterations were assessed using the following open-access software: PolyPhen-2,17 Sorting Tolerant From Intolerant (SIFT),18 and Mutation taster.19
The effect on splicing of LDLR putative splice site variants was assessed using Splice-Site Predictor (Splice Port),20 Neural Network Splice Site Prediction Tool (NNSSP),21 and Neural Network Predictions of Splice Sites in Humans (NetGen2).22
Functional assays of LDLR variants: c.-13A>G, c.818-3C>G, and c.1706-10G>A
Total messenger RNA was obtained from blood mononuclear cells freshly isolated from the patients carrying these variants. Functional assays were performed as described before.9,23
Site-directed mutagenesis, transfection, and Western blot analysis
The oligonucleotides used to generate the different plasmids carrying the LDL receptor variants under study are presented in the Supplementary Methods online as well as the mutagenesis protocol. Cell transfection and semiquantitative immunoblotting were performed as described before.24
LDL isolation and lipoprotein labeling
LDL was isolated from 3 ml serum samples from healthy individuals using two-step centrifugation (Supplementary Methods online). LDL was labeled with fluorescein isothiocyanate as previously described.25
Quantification of LDLR expression and activity by flow cytometry
LDLR cell surface expression and LDL binding and uptake were determined by flow cytometry, specifically fluorescence-activated cell sorting, as previously described.24
Confocal laser scanning microscopy
Confocal laser scanning microscopy was used to analyze LDLR expression and intracellular colocalization of LDLR, as described before24 (Supplementary Methods online).
Kinetics of LDLR variants
Expression at different incubation times in the presence of LDL was measured, and studies of LDL–LDLR binding at different pH values were performed, as previously described.26
Statistical analysis
Statistical analysis of the lipid profile was performed using SPSS software (version 17.0 for Windows; SPSS, Chicago, IL). Frequencies of qualitative variables were compared using the χ2 test. Mean values of quantitative variables were compared using the Student t test or analysis of variance for independent data, whereas median values were compared with the nonparametric Mann–Whitney or Kruskal Wallis median tests.
For functional assay studies, all measurements were performed at least three times, with n = 3 unless otherwise stated. Results are presented as mean ± SD. Levels of significance were determined using a two-tailed Student t test. A P-value <0.05 was considered statistically significant.
Results
In the Portuguese FH study cohort, about 35 variants (30%) have an uncertain pathogenic effect. In an effort to continue the characterization of all these variants, eight LDLR alterations were chosen for this study based on the following criteria: (i) the prevalence in our cohort (Supplementary Figure S1 online), (ii) lack of cosegregation, (iii) the severity of the phenotype, and (iv) sample availability for functional studies.
In silico analysis
The results obtained by different software packages are presented in Tables 1 and 2 .
Splicing and promoter alterations assays
Functional studies of alterations c.-13A>G and c.1706-10G>A revealed that both are nonfunctional, whereas c.818-3C>G is a functional alteration leading to the retention of two nucleotides in intron 5 (Supplementary Figure S2 online). The promoter sequence variant was classified as nonpathogenic because both alleles (T/C) of the LDLR single nucleotide polymorphism rs2228671 were present in patients’ messenger RNA, indicating that the variant does not affect messenger RNA expression. We also verified that both alleles of the same single nucleotide polymorphism (SNP) were present in a patient with the c.1706-10G>A alteration, and there was no intron retention or exon skipping.
Expression of LDLR variants in CHO-ldlA7 cells
Only one band was detected for wild-type (wt) LDLR and the p.Gly76Trp variant, corresponding to the mature form (apparent molecular weight 130 kDa) (Supplementary Figure S3 online, lanes 1 and 2). Two bands were detected for the p.Arg406Trp variant, though the precursor form of the protein is reduced (Supplementary Figure S3 online, lane 3). Finally, p.Ile441Thr, p.Gly545Trp, and p.Cys698Phe LDLR variant expression was detected only as the precursor form (Supplementary Figure S3 online, lanes 4, 5 and 6). Equal loading of protein was confirmed in each blot by membrane stripping and further incubation with antibodies to visualize cytosolic GAPDH protein (Supplementary Figure S3a online). The extent of protein expression was determined by quantitative densitometric analysis (Supplementary Figure S3b online). No statistical differences in expression were observed among the LDLR constructs, as shown in Supplementary Figure S3b online.
Functional study of the LDLR variants by fluorescence-activated cell sorting
As shown in Figure 1a , only p.Gly76Trp shows LDLR expression at cell surface, similar to the wt (wt: 100 ± 5; p.Gly76Trp: 102 ± 2 (arbitrary units)). Expression of p.Arg406Trp, p.Ile441Thr, p.Gly545Trp, and p.Cys698Phe was significantly reduced compared with the wt LDLR (p.Arg406Trp: 65 ± 5; p.Ile441Thr: 7 ± 5; p.Gly545Trp: 7 ± 5; p.Cys698Phe: 8 ± 6; P < 0.01). Binding of p.Gly76Trp was similar to wt binding (wt: 100 ± 3; p.Gly76Trp: 90 ± 3); however, the binding activities of the other four variants analyzed were diminished when compared with wt (p.Arg406Trp: 60 ± 4; p.Ile441Thr: 5 ± 3; p.Gly545Trp: 6 ± 4; p.Cys698Phe: 3 ± 1; P < 0.01) ( Figure 1b ). As shown in Figure 1c , and in agreement with LDLR expression and binding results, LDL internalization by p.Gly76Trp is similar to wt (wt: 100 ± 4; p.Gly76Trp: 95 ± 5), and the LDL uptake determined for the other four variants is significantly diminished compared with wt (p.Arg406Trp: 62 ± 4; p.Ile441Thr: 10 ± 3; p.Gly545Trp: 11 ± 4; p.Cys698Phe: 8 ± 5; P < 0.01). According to the results obtained, it can be concluded that p.Gly76Trp is a nonpathogenic LDLR variant, whereas p.Arg406Trp is a pathogenic variant with diminished activity (≈40%), and p.Ile441Thr, p.Gly545Trp, and p.Cys698Phe are pathogenic with a near complete loss of activity (≈10%).
Functional characterization of LDLR variants in CHO- ldlA7 transfected cells. (a) LDLR expression at the cellular membrane. (b) Low-density lipoprotein (LDL)–LDLR binding after 4 h incubation at 4 °C. (c) LDL internalization efficiency after 4 h incubation at 37 °C. Cells (n = 10,000) were acquired in a Facscalibur cytometer, and values of LDL uptake and binding and LDLR expression were calculated as described in the Methods. The values represent the mean of triplicate determinations (n = 3); error bars represent ±SD. *P < 0.001 compared with wild type using the Student t test.
Determination of LDLR class mutation by confocal microscopy
According to the results obtained by fluorescence-activated cell sorting, p.Arg406Trp is most probably a class 2b (LDLR partially retained in the endoplasmic reticulum) or class 5 (impaired recycling of LDLR), and p.Ile441Thr, p.Gly545Trp, and p.Cys698Phe are probably class 2a (total retention of LDLR in the endoplasmic reticulum). As shown in Figure 2 , wt does not colocalize with calregulin, residing mostly at the cellular membrane. However, p.Arg406Trp LDLR showed partial colocalization with the endoplasmic reticulum, indicating that it belongs to class 2b. p.Ile441Thr, p.Gly545Trp, and p.Cys698Phe LDLR variants colocalize almost completely with calregulin and thus are classified as class 2a variants.
Analysis of wild type, p.Arg406Trp, p.Ile441Thr, p.Gly545Trp, and p.Cys698Phe LDLR by confocal microscopy. (a) LDLR expression and colocalization with calregulin, a specific endoplasmic reticulum marker, was determined 48 h after cell transfection. Immunostaining was performed with anti-hLDLR and anti-calregulin antibodies. Texas Red– and Alexa Fluor 488–labeled secondary antibodies were used to visualize LDLR and calregulin, respectively. The images show a representative individual cell (n = 30). (b) The relative amount of LDLR (red) and calregulin (green) and the percentage of LDLR to calregulin colocalization (orange). The histograms represent the mean ± SD (n = 30 cells). *P < 0.001 compared with wild type using the Student t test.
Kinetics of p.Arg406Trp LDLR expression
Kinetic studies were performed to exclude any possibility of an impaired defect in the recycling of p.Arg406Trp LDLR. Expression of LDLR at the cell surface was determined by fluorescence-activated cell sorting upon the addition of LDL, and the percentage of LDLR relative to time 0 (without LDL) was determined at each incubation time. As shown in Supplementary Figure S4a online, expression of wt LDLR does not diminish after the addition of LDL during incubation. Expression of p.Arg406Trp mutant is also not decreased over time during incubation with LDL (wt: 100 ± 4; p.Arg406Trp: 65 ± 4 at time 0). As an internal control, the previously characterized class 5 variant p.Arg416Trp26 was used to illustrate the decrease of LDLR expression at the cell membrane occurring as a result of impaired recycling. We also mimicked the acid-dependent mechanism of lipoprotein release that occurs in the endosomal compartment upon acidification. As shown in Supplementary Figure S4b online, LDL–LDLR binding for wt was dependent on pH, being 75% less efficient at pH 5.5 compared with pH 7.5. Similar results were determined for the p.Arg406Trp mutant, indicating that there is no impaired release of LDL/LDLR upon endosomal acidification, confirming that this variant is not a class 5 LDLR mutation. By contrast, for the positive control p.Arg416Trp LDLR, LDL release at an acidic pH was not as efficient as in wt, resulting in binding 37% lower at pH 5.5 than at pH 7.5 (Supplementary Figure S4b online).
Lipid profile of functional and nonfunctional alterations
To increase the accuracy of our analysis, we choose to include the results of five nonpathogenic (neutral) variants previously reported (p.Gly269Asp, p.Glu277Lys, c-1061-8T>C, c.2140+5G>A, and p.Val859Met).23,27,28,29
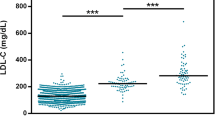
Biochemical characteristics of each mutation carrier are shown in Supplementary Table S1 online. Only data for adults without treatment were included (except lipoprotein(a)) because no values were registered for the majority of pediatric patients. When the phenotype of functional alteration carriers and neutral alteration carriers were compared, carriers of functional alterations presented a severe phenotype; the differences were statistically significant for all parameters except high-density lipoprotein cholesterol and apoA1, as well as age (Supplementary Table S1 online). Also, the mean values of LDL-C percentile were significantly higher in the carriers of functional alterations than in those identified with neutral alterations (83.6 ± 22.4 vs. 61.2 ± 34.2 mg/dl; P = 0.002) (Supplementary Table S1 online).
A family history of premature cardiovascular disease was more common in carriers of functional alterations (74.2%) than in carriers of neutral alterations (29.2%) (Supplementary Table S2 online). The same was observed in patients with LDL-C values above the 75th percentile (92.5% vs. 61.1%; P < 0.001). For index patients, however, there were no statistically significant differences concerning LDL-C values above the 95th percentile (75.9% vs. 79.2%; P = 0.520) (Supplementary Table S2 online).
Because the p.Arg406Trp alteration presented a decrease in protein activity (close to borderline) of only 40%, the phenotype of individuals with this alteration was compared with that of carriers of functional alterations and neutral alterations ( Table 3 ). Individuals with p.Arg406Trp presented a more severe phenotype when compared with carriers of neutral alterations for total cholesterol, LDL-C, apoB, apoB/apoA1 ratio, and LDL-C percentile mean values (333.9 ± 78.5 vs. 278.2 ± 59.23 mg/dl (P = 0.02); 237.7 ± 112.4 vs. 168.2 ± 53.0 mg/dl (P = 0.002); 144.5 ± 44.3 vs. 103.0 ± 28.2 mg/dl (P = 0.002); 0.87 ± 0.29 vs. 0.63 ± 0.28 mg/dl (P = 0.027); and 82.4 ± 22.8 vs. 61.2 ± 34.2 (P = 0.03), respectively). When compared with carriers of functional alterations, however, a statistically significant difference was not found, although the mean levels of total cholesterol, LDL-C, apoB, and apoB-to-apoA1 ratio were higher for p.Arg406Thr (333.9 ± 78.5 vs. 322.2 ± 68.2 mg/dl (P = 0.82); 237.7 ± 112.4 vs. 230.7 ± 94.1 mg/dl (P = 0.96); 144.5 ± 44.3 vs. 117.4 ± 35.4 mg/dl (P = 0.19); 0.87 ± 0.29 vs. 0.79 ± 0.21 mg/dl (P = 0.492); 82.4 ± 22.8 vs. 84.4 ± 22.4 (P = 0.73)) ( Table 3 ). Triglycerides, high-density lipoprotein cholesterol, and ApoA1 values were similar between these three groups. The cosegregation data are very similar between p.Arg406Thr and functional carriers, and carriers of neutral variants present lower cosegregation rates, showing a high percentage of nonaffected individuals with neutral variants. The analysis of LDL-C percentiles, however, presented contradictory results: p.Arg406Thr carriers showed the lowest rates for index cases, with LDL ≥75th percentile and LDL ≥95th percentile.
Discussion
A detailed analysis of the phenotype and genotype presented by 55 families with clinical diagnosis of FH and 13 different alterations in the LDLR was conducted. The aim of this investigation was to highlight the importance of the integrated analysis of clinical, molecular, and functional data for the correct diagnosis of FH. Of these 13 alterations, 7 have been described in other populations as well as in a Portuguese FH cohort. All variants, except two that are novel, are described in the FH database as putative mutations causing disease, and eight did not have functional studies. For this reason, an extensive functional characterization of these eight LDLR alterations was performed, allowing the functional assessment of these variants. Pathogenicity status was attributed to five alterations, and three had a neutral effect on protein activity. To increase accuracy, data on five neutral alterations with functional characterization, and found before in different populations, were included in the analysis. Carriers of neutral alterations presented a significantly lower incidence of premature cardiovascular disease, lower concentrations of atherogenic lipoproteins, and also a lower LDL-C percentile than functional alterations carriers. The cosegregation of the variants with the hypercholesterolemia phenotype was less well established for neutral variants; only 78.7% of the hypercholesterolemic subjects were alteration carriers, and 87.5% of normolipidemic individuals presented these alterations compared with 96.3% and 0%, respectively, for functional alteration carriers. Deciding which alterations are pathogenic based on the lipid profile is not straightforward, however, and if there are no data of hypercholesterolemic or normolipidemic individuals within the same family, performing cosegregation studies is also not possible. Only when data of at least 50 alteration carriers are known is it possible to use cosegregation to assess a variant’s pathogenicity,13 and usually this is not an easy requirement to fulfill in the majority of FH cohorts. In silico analysis was also not conclusive for the majority of the alterations, having assessed correctly only 7/13 alterations. So, functional study is essential to determine a variant’s pathogenicity, and these studies can be performed by any research laboratory with access to a flow cytometer and a confocal microscopy since the protocol has been published,24 or collaborations can be established. Nevertheless, when the lipid and molecular profiles of affected and nonaffected relatives are known, this information is useful for the pathogenicity assessment of a variant, and it should always be taken into consideration.
The importance of this integrated analysis of clinical, molecular, and functional data is demonstrated by the assessment of p.Arg406Thr, a mutation described worldwide but for which functional assays have not been performed. The lipid profile and cosegregation analysis of p.Arg406Thr carriers and the other functional mutations carriers under study are similar and a statistically significant exists between these and carriers of neutral alterations for all atherogenic particles except high-density lipoprotein cholesterol, apoA1, and triglycerides. This indicates that this alteration is probably a disease-causing mutation. When the LDL-C percentiles were analyzed, however, the results were not as straightforward; the prevalence of index cases with the alterations and with LDL-C ≥75th percentile and LDL-C ≥95th percentile was lower for p.Arg406Thr (although without statistical significance) than for index carriers of the neutral or functional alterations. For relatives, the lower prevalence was seen for neutral alterations carriers but without significant differences. In conclusion, this phenotype/genotype analysis was not able to produce a valid pathogenicity assessment for p.Arg406Thr. When the functional study was performed, the LDLR activity was determined and the reason for the oscillation in the lipid profile was understood; this variant retained 60% of LDLR activity concerning expression, binding, and internalization. This allows a total activity of the LDLR receptor of 80% (assuming that the nonmutated allele produces 50% of the active protein) compared with 50–55% that is seen for a null mutation or for the mutations characterized in this study (p.Ile442Thr, p.Gly545Trp, and p.Cys698Phe). By contrast, p.Gly76Trp—classified here as neutral—presented a total activity of about 95% for binding and internalization. Because the p.Arg406Thr variant retains 60% activity, it can be considered a mild mutation, and the variation in the phenotype of carriers can be attributed to environmental factors that are known to affect the phenotype, even in patients with FH.30 The cutoff value for determining whether an LDLR variant is considered a functional mutant by in vitro studies has not been established, but, based on several published studies,1,27,29,31,32,33 in vitro LDLR activity less than 70–80% (either in expression, binding, or internalization), corresponding to 85–90% total LDLR activity, could classify a variant as pathogenic.
The functional classification of a variant is also important for patient management so patients can be advised according to their condition. Because carriers of these neutral alterations present a milder phenotype, most probably do not have FH and therefore need different counseling and treatment approaches to tackle their dyslipidemia. The results obtained for p.Arg406Thr can also have a clinical implication; these patients probably need a less aggressive medication to control their LDL-C concentrations because their mutant LDLR still retains some activity (60% for the mutated allele, 80% in total). This way the determination of the variant functionality can be a step forward for the personalized treatment of patients with FH.
The integrated analysis presented here is important for the correct assessment of patients with FH who might otherwise be misdiagnosed. A detailed analysis of the protein at the molecular level, adding to the clinical and molecular data already obtained routinely, provides information relevant to understanding the phenotype observed in these patients and that can be translated into clinical management improvements.
Disclosure
The authors declare no conflict of interest.
References
Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat 1992;1:445–466.
Fouchier SW, Defesche JC, Umans-Eckenhausen MW, Kastelein JP. The molecular basis of familial hypercholesterolemia in The Netherlands. Hum Genet 2001;109:602–615.
Humphries SE, Cranston T, Allen M, et al. Mutational analysis in UK patients with a clinical diagnosis of familial hypercholesterolaemia: relationship with plasma lipid traits, heart disease risk and utility in relative tracing. J Mol Med (Berl) 2006;84:203–214.
Goldstein JL, Hobbs HH, Brown MS. Familial Hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease, 7th edn. McGraw-Hill: New York, NY, 1995:1981–2030.
DeMott K, Nherera L, Shaw E, et al. Clinical Guidelines and Evidence Review for Familial Hypercholesterolaemia: The Identification and Management of Adults and Children with Familial Hypercholesterolaemia. National Collaborating Centre for Primary Care and Royal College of General Practitioners: London, UK, 2008.
Alves AC, Medeiros AM, Francisco V, Gaspar IM, Rato Q, Bourbon M. Molecular diagnosis of familial hypercholesterolemia: an important tool for cardiovascular risk stratification. Rev Port Cardiol 2010;29:907–921.
European Association for Cardiovascular Prevention & Rehabilitation; Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011; 32: 1769–1818.
Usifo E, Leigh SE, Whittall RA, et al. Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Ann Hum Genet 2012;76:387–401.
Investigators of the Portuguese FH Study; Medeiros AM, Alves AC, Francisco V, Bourbon M. Update of the Portuguese Familial Hypercholesterolaemia Study. Atherosclerosis 2010;212:553–558.
Kusters DM, Huijgen R, Defesche JC, et al. Founder mutations in the Netherlands: geographical distribution of the most prevalent mutations in the low-density lipoprotein receptor and apolipoprotein B genes. Neth Heart J 2011;19:175–182.
Investigators of Portuguese FH Study; Bourbon M, Alves AC, Medeiros AM, Silva S, Soutar AK. Familial hypercholesterolaemia in Portugal. Atherosclerosis 2008;196:633–642.
Fouchier SW, Kastelein JJ, Defesche JC. Update of the molecular basis of familial hypercholesterolemia in The Netherlands. Hum Mutat 2005;26:550–556.
Huijgen R, Kindt I, Defesche JC, Kastelein JJ. Cardiovascular risk in relation to functionality of sequence variants in the gene coding for the low-density lipoprotein receptor: a study among 29,365 individuals tested for 64 specific low-density lipoprotein-receptor sequence variants. Eur Heart J 2012;33:2325–2330.
Gómez-Gerique JA, Gutiérrez-Fuentes JA, Montoya MT, et al. Lipid profile of the Spanish population: the DRECE (diet and risk of cardiovascular disease in Spain) study. DRECE study group. Med Clin (Barc) 1999;113:730–735.
Rato Q. Terapêutica Farmacológica das Dislipidemias. Rev Port Cardiol 2010;29(suppl. III):49–66.
NHLBI Grand Opportunity Exome Sequencing Project; Lange LA, Hu Y, Zhang H, et al. Whole-exome sequencing identifies rare and low-frequency coding variants associated with LDL cholesterol. Am J Hum Genet 2014;94:233–245.
Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010;7:248–249.
Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 2003;31:3812–3814.
Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 2014;11:361–362.
Dogan RI, Getoor L, Wilbur WJ, Mount SM. SplicePort – an interactive splice-site analysis tool. Nucleic Acids Res 2007;35(Web Server issue):W285–W291.
Reese MG, Eeckman FH, Kulp D, Haussler D. Improved splice site detection in Genie. J Comput Biol 1997;4:311–323.
Hebsgaard SM, Korning PG, Tolstrup N, Engelbrecht J, Rouzé P, Brunak S. Splice site prediction in Arabidopsis thaliana pre-mRNA by combining local and global sequence information. Nucleic Acids Res 1996;24:3439–3452.
Bourbon M, Duarte MA, Alves AC, Medeiros AM, Marques L, Soutar AK. Genetic diagnosis of familial hypercholesterolaemia: the importance of functional analysis of potential splice-site mutations. J Med Genet 2009;46:352–357.
Etxebarria A, Benito-Vicente A, Alves AC, Ostolaza H, Bourbon M, Martin C. Advantages and versatility of fluorescence-based methodology to characterize the functionality of LDLR and class mutation assignment. PLoS One 2014;9:e112677.
Dardik R, Varon D, Tamarin I, et al. Homocysteine and oxidized low density lipoprotein enhanced platelet adhesion to endothelial cells under flow conditions: distinct mechanisms of thrombogenic modulation. Thromb Haemost 2000;83:338–344.
Etxebarria A, Benito-Vicente A, Palacios L, et al. Functional characterization and classification of frequent low-density lipoprotein receptor variants. Hum Mutat 2015;36:129–141.
Etxebarria A, Palacios L, Stef M, et al. Functional characterization of splicing and ligand-binding domain variants in the LDL receptor. Hum Mutat 2012;33:232–243.
Ekström U, Abrahamson M, Sveger T, Lombardi P, Nilsson-Ehle P. An efficient screening procedure detecting six novel mutations in the LDL receptor gene in Swedish children with hypercholesterolemia. Hum Genet 1995;96:147–150.
Silva S, Alves AC, Patel D, Malhó R, Soutar AK, Bourbon M. In vitro functional characterization of missense mutations in the LDLR gene. Atherosclerosis 2012;225:128–134.
Pimstone SN, Sun XM, du Souich C, Frohlich JJ, Hayden MR, Soutar AK. Phenotypic variation in heterozygous familial hypercholesterolemia: a comparison of Chinese patients with the same or similar mutations in the LDL receptor gene in China or Canada. Arterioscler Thromb Vasc Biol 1998;18:309–315.
Løhne K, Urdal P, Leren TP, Tonstad S, Ose L. Standardization of a flow cytometric method for measurement of low-density lipoprotein receptor activity on blood mononuclear cells. Cytometry 1995;20:290–295.
Urdal P, Leren TP, Tonstad S, Lund PK, Ose L. Flow cytometric measurement of low density lipoprotein receptor activity validated by DNA analysis in diagnosing heterozygous familial hypercholesterolemia. Cytometry 1997;30:264–268.
Ekstrom U, Abrahamson M, Sveger T, Sun XM, Soutar AK, Nilsson-Ehle P. Expression of an LDL receptor allele with two different mutations (E256K and I402T). Mol Pathol 2000;53:31–36.
Mozas P, Castillo S, Tejedor D, et al. Molecular characterization of familial hypercholesterolemia in Spain: identification of 39 novel and 77 recurrent mutations in LDLR. Hum Mutat 2004;24:187.
Pereira E, Ferreira R, Hermelin B, et al. Recurrent and novel LDL receptor gene mutations causing heterozygous familial hypercholesterolemia in La Habana. Hum Genet 1995;96:319–322.
Reshef A, Nissen H, Triger L, et al. Molecular genetics of familial hypercholesterolemia in Israel. Hum Genet 1996;98:581–586.
Khamis A, Palmen J, Lench N, et al. Functional analysis of four LDLR 5’UTR and promoter variants in patients with familial hypercholesterolaemia. Eur J Hum Genet 2014. doi:10.1038/ejhg.2014.199. e-pub ahead of print.
Jensen HK, Jensen LG, Hansen PS, Faergeman O, Gregersen N. High sensitivity of the single-strand conformation polymorphism method for detecting sequence variations in the low-density lipoprotein receptor gene validated by DNA sequencing. Clin Chem 1996;42(8 Pt 1):1140–1146.
Heath KE, Gudnason V, Humphries SE, Seed M. The type of mutation in the low density lipoprotein receptor gene influences the cholesterol-lowering response of the HMG-CoA reductase inhibitor simvastatin in patients with heterozygous familial hypercholesterolaemia. Atherosclerosis 1999;143:41–54.
Acknowledgements
The authors thank Rocío Alonso for excellent technical assistance. They are also grateful to A. Gómez-Muñoz for making flow cytometry facilities available and SGIker (Analytical and High-Resolution Microscopy in Biomedicine Service of the UPV/EHU) and Monty Krieger for kindly providing CHO-ldlA7 cells. Capillary sequencing was performed at Unidade de Tecnologia e Inovação (Departamento de Genética Humana, Instituto Nacional de Saúde Doutor Ricardo Jorge). Funding was obtained from the Portuguese Science and Technology Foundation (PTDC/SAU-GMG/101874/2008), the Spanish Ministry of Economy and Competitiveness (grant BFU 2012–36241), and Programa INNPACTO (grant IPT-2011-0817-010000). A.C.A. was supported by a PhD student grant (SFRH/BD/27990/2006) and a research grant from PTDC/SAU-GMG/101874/2008.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
(ZIP 796 kb)
Rights and permissions
About this article
Cite this article
Benito-Vicente, A., Alves, A., Etxebarria, A. et al. The importance of an integrated analysis of clinical, molecular, and functional data for the genetic diagnosis of familial hypercholesterolemia. Genet Med 17, 980–988 (2015). https://doi.org/10.1038/gim.2015.14
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2015.14
Keywords
This article is cited by
-
Mutation type classification and pathogenicity assignment of sixteen missense variants located in the EGF-precursor homology domain of the LDLR
Scientific Reports (2020)
-
The complex molecular genetics of familial hypercholesterolaemia
Nature Reviews Cardiology (2019)
-
p.(Asp47Asn) and p.(Thr62Met): non deleterious LDL receptor missense variants functionally characterized in vitro
Scientific Reports (2018)