Abstract
Purpose: Pharmacogenetic testing is one of the primary drivers of personalized medicine. The use of pharmacogenetic testing may provide a lifetime of benefits through tailoring drug dosing and selection of multiple medications to improve therapeutic outcomes and reduce adverse responses. We aimed to assess public interest and concerns regarding sharing and storage of pharmacogenetic test results that would facilitate the reuse of pharmacogenetic data across a lifetime of care.
Methods: We conducted a random-digit-dial phone survey of a sample of the US public.
Results: We achieved an overall response rate of 42% (n = 1139). Most respondents indicated that they were extremely or somewhat comfortable allowing their pharmacogenetic test results to be shared with other doctors involved in their care management (90% ± 2.18%); significantly fewer respondents (74% ± 3.27%) indicated that they were extremely or somewhat comfortable sharing results with their pharmacist (P < 0.0001).
Conclusion: Patients, pharmacists, and physicians will all be critical players in the pharmacotherapy process. Patients are supportive of sharing pharmacogenetic test results with physicians and pharmacists and personally maintaining their test results. However, further study is needed to understand which options are needed for sharing, appropriate storage, and patient education about the relevance of pharmacogenetic test results to promote consideration of this information by other prescribing practitioners.
Similar content being viewed by others
Main
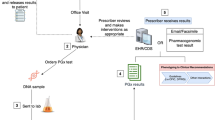
Pharmacogenetic (PGx) testing, or the use of genetic tests to determine the optimal pharmaceutical therapy for a given individual, is considered to be one of the most promising early clinical applications arising from genomics research, with the potential to reduce the prevalence of adverse drug responses and improve efficacy.1,2 A number of drugs are metabolized by a handful of highly polymorphic cytochrome P-450 liver enzymes.3 Over a person's lifetime, they are likely to be prescribed several medications for which these and other genes have an important role in determining the rate of metabolism. Thus, the results of a PGx test will be pertinent not only to the immediate clinical situation for which testing is initially ordered but also likely for future clinical encounters. As we enter the PGx era, it is imperative to consider how relevant PGx results should be managed with respect to storage and access to minimize information fragmentation and duplicate testing.4 As PGx testing assay costs and their cost-effectiveness for a single clinical decision is a major barrier to the routine clinical application of PGx, the ability to reuse PGx data across a lifetime of care could significantly facilitate the use of such testing in routine practice.
As part of a national survey exploring public interest and attitudes toward PGx testing and specifically, ancillary information revealed by PGx testing, we asked respondents about sharing and storage of PGx test results. To our knowledge, no studies have examined this important issue essential to ensuring the lifetime benefits of PGx testing. In this article, we discuss the benefits and limitations of several options of storage and access of PGx results informed by findings from a national public survey on PGx testing on sharing results with other physicians and pharmacists and patient management of results.
MATERIALS AND METHODS
Survey development and administration
As described elsewhere,5 we developed a survey to explore public attitudes regarding PGx testing and the potential for ancillary information. Specifically, the final survey comprised several sections including personal and family experience with medications, interest in PGx testing given certain risks and uses of testing, sharing and management of test results, and attitudes toward management of ancillary information revealed by PGx testing. We report in this study data regarding public attitudes toward sharing and management of test results (see Haga et al., in press and Haga et al., in press for other survey findings).5,6
The survey was first piloted on a random sample of the local North Carolina population before launching the national survey in fall 2009. A random-digit-dial sample of telephone numbers in the continental US was selected for the national survey and stratified by US census regions to ensure representativeness. Eligibility was based on reaching a household with an English-speaking resident, aged 18 years or older. If more than one eligible adult resided in the household, one was randomly selected.
Data analysis
Descriptive statistical analysis was conducted to examine respondent demographics and attitudes toward sharing and management of PGx test results. For logistic regression analyses, model building was based on hypothetically related covariates with adjustment for demographic characteristics; final variable selection was conducted using the backward selection approach. Odds ratios and corresponding 95% confidence intervals were computed; a significance level of 0.05 was used for all statistical tests. To adjust for control variables, Cochran-Mantel-Haenszel test was applied when comparing two groups on a binary response. All analyses were conducted in SAS (Version 9.1.3 using Proc Frequency, Proc Logistic & Proc Regression).
RESULTS
A response rate of 42%7 was achieved in this survey. Because respondents tended to be older (51% were aged 55 years or older), white (86%), and female (61%) to a greater extent than would be expected by chance alone, the survey data were adjusted by age (18–34 years, 35–54 years, and 55 years and older), race (white and non-white), and gender based on normative data from the 2008 American Community Survey.8 After poststratification, the adjusted sample better reflected the US population (51% female, 78% white, and 31% aged 55 years or older). To further reduce the potential effect of bias, nonresponse adjustments were predicated on US census regions.
After assessing respondents' likelihood to undergo PGx testing given a range of uses and risks, we asked respondents to indicate their level of comfort regarding sharing and management of their PGx results. In response to a question about their level of comfort in allowing test results to be shared with their other doctors, most respondents indicated that they were extremely or somewhat comfortable allowing their PGx test results to be shared with other doctors involved in their care management (90% ± 2.18%). In addition, it was found that respondents who had excellent/good health were less likely to be comfortable sharing results with other doctors (odds ratio = 0.45, P = 0.007, confidence interval [0.25–0.80]).
When asked about their level of comfort regarding sharing of their PGx test result with a pharmacist, 74% (±3.27%) of respondents indicated that they were extremely or somewhat comfortable sharing the results with their pharmacist. There were no respondent variables found to be significantly associated with level of comfort regarding sharing results with a pharmacist. However, after adjusting for sex, age group, level of education, and race, there was a statistically significant difference in the proportion of respondents who indicated that they were comfortable sharing their results with other doctors and the proportion of respondents who were comfortable sharing their results with their pharmacist (Cochran-Mantel-Haenszel statistic = 144.70, P < 0.0001).
When asked about how comfortable they would be keeping their PGx results in a personal record, such as stored on a card kept in the patient's wallet or purse, 70% (±3.54%) of respondents felt extremely or somewhat comfortable with this option for data storage. No respondent variables were associated with level of comfort in keeping their PGx results.
DISCUSSION
The problems associated with medical information fragmentation have been demonstrated in both primary care and specialty settings,9–11 potentially resulting in adverse outcomes or delay of treatment.10 In addition, missing medical information could lead to duplicate testing,4 potentially resulting in delayed treatment and wasted resources. As indicated by our survey findings, the public seems comfortable having their PGx results shared with their treating physician(s), which would help avoid duplicate testing. To promote sharing of results during this early stage of use of PGx testing, it will be necessary to emphasize to patients the importance of these results for any future medical treatments prescribed and the need to inform new treating physicians about their results. In addition, physicians should begin to ask patients about previous PGx testing that may inform treatment. Similar to routinely asked questions regarding drug allergies, we could envision patients routinely queried about PGx testing for P450 enzymes and classification as a poor metabolizer, intermediate metabolizer, or ultrarapid metabolizer. However, given that self-reported drug allergies are often inaccurate, leading to unnecessary avoidance of drugs,12–14 expecting patients to accurately recall and report their CYP metabolic status, much less their genotype, may be overly ambitious until the public becomes more knowledgeable about testing and the type of information provided by the test. Therefore, options are needed for appropriate storage and patient education about the relevance of PGx test results to promote consideration of this information by other prescribing practitioners. We speculate that healthy respondents may be less likely to be comfortable sharing results with other physicians due to concerns about privacy: their perceived benefit from sharing this private information may be less than the perceived costs, e.g., with respect to potential life insurance discrimination.
Haga and Burke15 proposed that PGx results be retained by either patients or stored in pharmacy databases to maximize consideration of PGx test results for new treatments. Errors in self-reporting and recall may be avoided if PGx test results were stored in a personal health record. The use of personal health records has been gradually expanding16 as is physician willingness to use such records,17 which may improve the quality of care.18 Given that respondents indicated they would be comfortable maintaining their PGx results, testing laboratories could enable results to be accessible to patients in addition to sending the results to the physician's office. For example, the genetic testing company Navigenics, which up until recently provided testing directly to consumers, presented PGx test results (test interpretation not genotype) in a card format to be shared with physicians, listing the target drug, their risk of side effect, and the implications of their result. Patients would then be responsible for providing the PGx results to new providers when drugs are prescribed. Particularly in acute care situations, if this information is stored electronically on an easily accessible physical device (e.g., an insurance card) or on a secure Web site, the information could be quickly retrieved and considered when testing is not otherwise feasible. However, approximately one third of participants of a PGx testing study indicated that they would not share their results with their physicians due to perceived physician disinterest, incompetence, or burden.19 Other potential barriers to patient sharing may include concerns about privacy, stigmatization, discrimination, coverage, and/or access to potential treatments.
Another option may be to provide access of PGx test results to pharmacists. Pharmacists already play an important role in assuring the safety of drug therapy by assessing potential adverse drug interactions when a new drug is prescribed and by providing information about appropriate substitutions for patients with drug allergies and concomitant medications that should be avoided. The pharmacist's scope of practice has expanded to incorporate identification of alternative therapies to reduce cost or increase safety, prescribing privileges, vaccination services, and management for patients with complex drug regimens.20–26 Therefore, monitoring PGx information to assure appropriate drug dosing is a natural extension of the role of pharmacists.27–29 Indeed, schools of pharmacy30–32 and continuing education programs33 have begun to recognize the importance of education on PGx testing. A handful of studies have begun to explore the role of pharmacists and the use of PGx testing.34,35
Despite the expanded scope of pharmacy practices, the structure of an appropriate collaborative partnership between pharmacists and physicians is not yet well defined,36–39 particularly with community pharmacists.40,41 Pharmacists' limited access to a patient's medical history and other test results will hinder their ability to determine the need for PGx testing and use of alternative medication. In addition, different business models of prescription filling (e.g., mail order, wholesale, and retail) may not be amenable to a collaborative role between physicians and pharmacists with respect to PGx testing.
Similar to drug allergy information, PGx test results could be routinely stored in a patient's pharmacy record. The majority of respondents indicated that they were willing to have their results shared with a pharmacist, although significantly fewer than those willing to have their results shared with other physicians, perhaps due to their limited relationship with pharmacists.42 Although pharmacists, along with physicians, have been ranked as the two most trusted sources of drug information by patients,43 the public's understanding of the services they may provide in addition to dispensing medication is limited,44,45 possibly attributing to the lower level of comfort indicated in our survey. In addition, potential patient concerns about privacy could further account for the lower level of comfort,46 particularly if patients' recognize that their results may be placed in an electronic pharmacy dispensing system. However, as we did not ask survey respondents about electronic data storage such as EHRs, it is still speculative what factors account for the differing levels of comfort.
In the event that a drug is ordered for which a PGx test is required or strongly recommended, a pharmacist may have an obligation to alert the prescribing physician about testing and/or confirm that the test was ordered. The potential of increased medical liability due to harms caused by failure to consider PGx testing has been considered in depth elsewhere,47 for which the defendants may include the drug manufacturer, insurance companies, physicians, and potentially pharmacists.
Although patients and pharmacists do play an important role in the medication process, the physician remains the central agent of most medication selection and prescribing. Thus, directly influencing physician prescribing practices will be critical for ensuring the appropriate consideration of PGx factors. In particular, electronic health records (EHRs) and their component subsystems (e.g., e-prescribing modules) provide ideal contexts for storing PGx data and using them to influence physician prescribing behaviors. Critical to such EHR-supported, PGx-enabled personalized prescribing will be the establishment of a national health information technology infrastructure that includes the use of common data and terminology standards and the establishment of up-to-date, clinically relevant knowledge resources for how PGx results should be used to guide clinical care.48,49 Although there are significant challenges to establishing such an infrastructure, an EHR-based approach to storing and using PGx results is highly promising. In particular, significant progress could be made if interoperability supportive of personalized pharmacotherapy is appropriately included as a core requirement in the federal government's current efforts to finance the widespread adoption of EHRs in the United States.50
Whether through personal health records, pharmacy information systems, or EHR systems, the routine provision of PGx guidance will require the widespread availability of rigorously curated knowledge on how patients' PGx test results should influence medication selection and dosing. Currently, commercial medication knowledge bases from companies such as First DataBank, Multum, and Medi-Span are widely integrated with pharmacy information systems and EHR systems to provide pharmacists and physicians with pharmacotherapy guidance. Thus, if one or more of these commercial offerings were to begin to incorporate PGx knowledge, and if PGx testing data were to be widely collected in a standardized manner, PGx could be incorporated into routine clinical practice through the leveraging of significant existing infrastructure and processes.
In moving forward, it will be important to keep in mind that the approaches we have outlined in this study are complementary rather than competitive. Patients, pharmacists, and physicians are all critical players in the pharmacotherapy process, and it will be important to acknowledge that the most effective approaches will likely involve a combination of such strategies as the use of personal health records, EHRs, and the pharmacy dispensing system with a database to track PGx results. Critical will be the use of common standards to enable interoperability across these various systems, as well as appropriate privacy and security safeguards to ensure that the wishes of patients are properly honored as PGx information becomes a more widely available and increasingly important consideration in the safe and effective prescribing of pharmacotherapies.
REFERENCES
Evans WE, McLeod HL . Pharmacogenomics—drug disposition, drug targets, and side effects. N Engl J Med 2003; 348: 538–549.
Shin J, Kayser SR, Langaee TY . Pharmacogenetics: from discovery to patient care. Am J Health Syst Pharm 2009; 66: 625–637.
Zhou SF, Liu JP, Chowbay B . Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 2009; 41: 89–295.
Stewart BA, Fernandes S, Rodriguez-Huertas E, Landzberg M . A preliminary look at duplicate testing associated with lack of electronic health record interoperability for transferred patients. J Am Med Inform Assoc 2010; 17: 341–344.
Haga SB, O'Daniel JM, Tindall GM, Lipkus IR, Agans R . Survey of US public attitudes toward pharmacogenetic testing. Pharmacogenomics J. [Published online ahead of print February 15, 2011].
Haga S, O'Daniel J, Tindall G, Lipkus I, Agans R . Public attitudes towards ancillary information revealed by pharmacogenetic testing under limited information conditions. Genet Med. [Published online ahead of print June 2, 2011].
The American Association for Public Opinion Research. Standard definitions: final dispositions of case codes and outcome rates for surveys, 7th ed. Lenexa, Kansas: AAPOR, 2011.
US Census Bureau. American Community Survey 2008, 2008. Available at: http://www.census.gov/acs/www/. Accessed May 2, 2011.
Gibby GL, Schwab WK . Availability of records in an outpatient preanesthetic evaluation clinic. J Clin Monit Comput 1998; 14: 385–391.
Smith PC, Araya-Guerra R, Bublitz C, et al. Missing clinical information during primary care visits. JAMA 2005; 293: 565–571.
Miller DW Jr, Yeast JD, Evans RL . Missing prenatal records at a birth center: a communication problem quantified. AMIA Annu Symp Proc 2005: 535–539.
Rebelo Gomes E, Fonseca J, Araujo L, Demoly P . Drug allergy claims in children: from self-reporting to confirmed diagnosis. Clin Exp Allergy 2008; 38: 191–198.
MacPherson RD, Willcox C, Chow C, Wang A . Anaesthetist's responses to patients' self-reported drug allergies. Br J Anaesth 2006; 97: 634–639.
Lutomski DM, Lafollette JA, Biaglow MA, Haglund LA . Antibiotic allergies in the medical record: effect on drug selection and assessment of validity. Pharmacotherapy 2008; 28: 1348–1353.
Haga SB, Burke W . Pharmacogenetic testing: not as simple as it seems. Genet Med 2008; 10: 391–395.
Ball MJ, Costin MY, Lehmann C . The personal health record: consumers banking on their health. Stud Health Technol Inform 2008; 134: 35–46.
Wynia MK, Torres GW, Lemieux J . Many physicians are willing to use patients' electronic personal health records, but doctors differ by location, gender, and practice. Health Aff (Millwood) 2011; 30: 266–273.
Council on Clinical Information Technology. Policy statement—using personal health records to improve the quality of health care for children. Pediatrics 2009; 124: 403–409.
Madadi P, Joly Y, Avard D, et al. Communicating pharmacogenetic research results to breastfeeding mothers taking codeine: a pilot study of perceptions and benefits. Clin Pharmacol Ther 2010; 88: 792–795.
Moczygemba LR, Goode JV, Gatewood SB, et al. Integration of collaborative medication therapy management in a safety net patient-centered medical home. J Am Pharm Assoc 2011; 51: 167–172.
Valgus J, Jarr S, Schwartz R, Rice M, Bernard SA . Pharmacist-led, interdisciplinary model for delivery of supportive care in the ambulatory cancer clinic setting. J Oncol Pract 2010; 6: e1–e4.
Westrick SC . Pharmacy characteristics, vaccination service characteristics, and service expansion: an analysis of sustainers and new adopters. J Am Pharm Assoc 2010; 50: 52–61.
Benavides S, Rodriguez JC, Maniscalco-Feichtl M . Pharmacist involvement in improving asthma outcomes in various healthcare settings: 1997 to present. Ann Pharmacother 2009; 43: 85–97.
Keely JL, American College of Physicians–American Society of Internal Medicine. Pharmacist scope of practice. Ann Intern Med 2002; 136: 79–85.
Hinthorn DR, Generali JA, Godwin HN . Pharmacist scope of practice: response to position paper. Ann Pharmacother 2002; 36: 718–720.
Clause S, Fudin J, Mergner A, et al. Prescribing privileges among pharmacists in Veterans affairs medical centers. Am J Health Syst Pharm 2001; 58: 1143–1145.
El-Ibiary SY, Cheng C, Alldredge B . Potential roles for pharmacists in pharmacogenetics. J Am Pharm Assoc 2008; 48: e21–e29; quiz e30–e32.
Roederer MW . Pharmacists and pharmacogenetics. Am J Health Syst Pharm 2009; 66: 1256–1257.
Clemerson JP, Payne K, Bissell P, Anderson C . Pharmacogenetics, the next challenge for pharmacy?. Pharm World Sci 2006; 28: 126–130.
Harirforoosh S, Fleckenstein L, Mahajan P, Aruoma OI, Huang Y, Moridani M . The importance of including topics related to pharmacogenetics, pharmacogenomics, and medical genetics in the pharmacy curriculum. Am J Pharm Educ 2009; 73: 114.
Murphy JE, Green JS, Adams LA, Squire RB, Kuo GM, McKay A . Pharmacogenomics in the curricula of colleges and schools of pharmacy in the United States. Am J Pharm Educ 2010; 74: 7.
Brazeau DA, Brazeau GA . A required course in human genomics, pharmacogenomics, and bioinformatics. Am J Pharm Educ 2006; 70: 125.
Brock TP, Faulkner CM, Williams DM, Smith SR . Continuing-education programs in pharmacogenomics for pharmacists. Am J Health Syst Pharm 2002; 59: 722–725.
Crews KR, Cross SJ, McCormick JN, et al. Development and implementation of a pharmacist-managed clinical pharmacogenetics service. Am J Health Syst Pharm 2011; 68: 143–150.
Zembles T . An inservice program on pharmacogenetics to individualize drug therapy. Am J Pharm Educ 2010; 74: 10.
Sellors J, Kaczorowski J, Sellors C, et al. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. CMAJ 2003; 169: 17–22.
Chen TF, de Almeida Neto AC . Exploring elements of interprofessional collaboration between pharmacists and physicians in medication review. Pharm World Sci 2007; 29: 574–576.
Monane M, Matthias DM, Nagle BA, Kelly MA . Improving prescribing patterns for the elderly through an online drug utilization review intervention: a system linking the physician, pharmacist, and computer. JAMA 1998; 280: 1249–1252.
Brown CA, Bailey JH, Lee J, Garrett PK, Rudman WJ . The pharmacist-physician relationship in the detection of ambulatory medication errors. Am J Med Sci 2006; 331: 22–24.
Alkhateeb FM, Unni E, Latif D, Shawaqfeh MS, Al-Rousan RM . Physician attitudes toward collaborative agreements with pharmacists and their expectations of community pharmacists' responsibilities in West Virginia. J Am Pharm Assoc 2009; 49: 797–800.
Pojskic N, Mackeigan L, Boon H, Ellison P, Breslin C . Ontario family physician readiness to collaborate with community pharmacists on drug therapy management. Res Social Adm Pharm 2011; 7: 39–50.
Keshishian F, Colodny N, Boone RT . Physician-patient and pharmacist-patient communication: geriatrics' perceptions and opinions. Patient Educ Couns 2008; 71: 265–284.
Donohue JM, Huskamp HA, Wilson IB, Weissman J . Whom do older adults trust most to provide information about prescription drugs?. Am J Geriatr Pharmacother 2009; 7: 105–116.
Law AV, Okamoto MP, Brock K . Perceptions of Medicare Part D enrollees about pharmacists and their role as providers of medication therapy management. J Am Pharm Assoc 2008; 48: 648–653.
Youmans SL, Schillinger D, Mamary E, Stewart A . Older African Americans' perceptions of pharmacists. Ethn Dis 2007; 17: 284–290.
Truong HA, Layson-Wolf C, de Bittner MR, Owen JA, Haupt S . Perceptions of patients on Medicare Part D medication therapy management services. J Am Pharm Assoc 2009; 49: 392–398.
Marchant GE, Milligan RJ, Wilhelmi B . Legal pressures and incentives for personalized medicine. Personalized Med 2006; 3: 391–397.
Scheuner MT, de Vries H, Kim B, Meili RC, Olmstead SH, Teleki S . Are electronic health records ready for genomic medicine?. Genet Med 2009; 11: 510–517.
Kawamoto K, Lobach DF, Willard HF, Ginsburg GS . A national clinical decision support infrastructure to enable the widespread and consistent practice of genomic and personalized medicine. BMC Med Inform Decis Mak 2009; 9: 17.
Centers for Medicare and Medicaid Services. Overview EHR Incentive Programs, 2011. Available at: http://www.cms.gov/ehrincentiveprograms. Accessed May 2, 2011.
Acknowledgements
This work was supported by the National Institutes of Health (R01 GM081416-01A1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: Dr. Haga is a member of the Patient Advisory and Public Policy Board of Generations Health, Inc.
Rights and permissions
About this article
Cite this article
Haga, S., Kawamoto, K., Agans, R. et al. Consideration of patient preferences and challenges in storage and access of pharmacogenetic test results. Genet Med 13, 887–890 (2011). https://doi.org/10.1097/GIM.0b013e31822077a5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e31822077a5
Keywords
This article is cited by
-
Precision medicine from a citizen perspective: a survey of public attitudes towards pharmacogenomics in Flanders
BMC Medical Genomics (2022)
-
Practical application of opt-out recruitment methods in two health services research studies
BMC Medical Research Methodology (2017)
-
Opinions, hopes and concerns regarding pharmacogenomics: a comparison of healthy individuals, heart failure patients and heart transplant recipients
The Pharmacogenomics Journal (2015)