Abstract
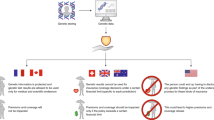
Genetic testing for heritable hearing loss involves a mix of patented and unpatented genes, mutations and testing methods. More than half of all hearing loss is linked to inherited mutations, and five genes are most commonly tested for in the United States. There are no patents on three of these genes, but Athena Diagnostics holds exclusive licenses to test for a common mutation in the GJB2 gene associated with about 50% of all cases as well as mutations in the MTRNR1 gene. This fragmented intellectual property landscape made hearing loss a useful case study to assess whether patent rights in genetic testing can proliferate or overlap, and whether it is possible to gather the rights necessary to perform testing. Testing for hearing loss is widely available, primarily from academic medical centers. Based on literature reviews and interviews with researchers, research on the genetics of hearing loss has generally not been impeded by patents. There is no consistent evidence of a premium in testing prices attributable to patent status. Athena Diagnostics has, however, used its intellectual property to discourage other providers from offering some tests. There is no definitive answer about the suitability of current patenting and licensing of commonly tested genes because of continuing legal uncertainty about the extent of enforcement of patent rights. Clinicians have also expressed concerns that multiplex tests will be difficult to develop because of overlapping intellectual property and conflict with Athena's sole provider business model.
Similar content being viewed by others
Main
Inherited DNA mutations account for over half of all hearing loss cases. Genetic hearing loss can be classified as “syndromic” or “nonsyndromic,” depending on whether there are associated clinical features (syndromic) or not (nonsyndromic). Mutations in a multitude of individual genes have been implicated in genetic hearing loss. In some cases, a single mutated gene is associated with hearing loss (dominant) and in others, symptoms occur when both parental genes an individual inherits are mutated (recessive) or a mutation occurs on the X chromosome (X-linked). Mutations in a few genes, GJB2/Connexin 26, GJB6/Connexin 30, SLC26A4/PDS, MTRNR1, and MTTS1, are most commonly tested in the United States.
Genetic testing for hearing loss can be controversial. Deafness and acquired hearing loss are disabilities, and whether or not to classify them as medical conditions is contested. Beliefs, lived experiences, and attitudes of individuals, both in the hearing and the Deaf Community differ widely. Whether genetic testing is useful or valuable is not a point of consensus. The complexities of when, whether, and how to classify deafness or hearing loss as a medical condition are beyond the scope of this case study. This case study is about testing for inherited mutations that can cause loss of hearing but with no particular view about whether such testing is valuable or whether it is a medical service.
The diverse perspectives on whether hearing loss is a disease or a disability influence consumer utilization of tests.1,2 This complicates the notion of “access,” because consumer values and preferences affect utilization. For those who deliberately choose not to use tests, lack of utilization does not indicate lack of access but rather expression of a choice. Although this is true in general for all genetic testing, the fact is that many in the Deaf Community contest the understanding of deafness as a disability is particularly relevant to this particular case study. Statistics on utilization are always only an indirect measure of access, but for hearing loss utilization rates are particularly suspect. Access is about how many people who want information and could benefit from it can get it; how hearing loss and deafness are regarded directly affects how many people actually want to know the cause, and consequently how many people want testing. Hereafter, our analysis will proceed on the assumption that we are addressing the use of genetic testing among those who want it and can benefit from it, while recognizing that some would not seek testing even if it were freely available at no cost, and access were not an issue.
Clinical guidelines from the American College of Medical Genetics (ACMG) recommend incorporating genetic testing into the diagnosis of congenital hearing loss.3 The benefits of genetic testing in diagnostic evaluation of hearing loss include:
-
1
Reducing additional time-consuming and expensive testing;
-
2
Facilitating early interventions such as hearing aids, cochlear implants, or sign language that significantly improve language ability;
-
3
Understanding disease progression;
-
4
Monitoring associated clinical manifestations and complications, particularly for syndromic hearing loss; and
-
5
Providing accurate information on the chance of recurrence that some may choose to use in making decisions about having children (and others may not).
PATENT ISSUES CONCERNING A MULTI-GENE, MULTI-MUTATION CONDITION
Hearing loss provides an opportunity to investigate how the patenting of different genes, mutations, and methods by multiple parties can affect access to genetic testing. Patents on multiple DNA sequences (both normal genes and mutations) owned by many parties have raised concerns about “patent thickets” or “anticommons.” An anticommons can occur when the intellectual property is dispersed, making it difficult to accumulate all the permissions needed, in this case to offer genetic tests for the mutations that cause hearing loss. This problem was characterized most famously in May 1998 by Heller and Eisenberg.4
The related notion of a patent thicket is that there is so much intellectual property that needs to be accumulated that it becomes difficult to cut through it all. This is a problem of density and profusion. The two concepts are distinct, but travel together in the real world, in areas where many patents have been granted to many players.
Another concern about patents is “blocking,” where a single patent owner with claims pertaining to common variants (or to a key method) can block others from doing genetic testing. Blocking can happen from just one or a few patents on key sequences, key methods, or other inventions, if they are difficult or impossible to invent around. This is a concern for hearing loss genes because patents on one or a few common variants might enable those who hold the relevant patents to prevent others from testing for other hearing loss genes.
One concept in intellectual property that requires aggregation of many patent rights is the incentive for hold-out. This was not highlighted in our case study, but is a possibility in the future, depending on how the tests evolve. The fact that different mutations have different frequencies (and therefore explain different fractions of cases) means that the potential commercial value of a mutation patent varies. Patents covering common variants should, therefore, generally be more valuable for clinical testing than rare ones. This makes patent pooling more complicated, because many pools simply count patents rather than try to weigh their value, and this may not work for genetic testing even if all the other issues about setting up patent pools were to get resolved. The hold-out incentive appears when a pool has started to form, but a key patent lies outside the pool, and the patent-holder perceives he or she has bargaining advantage and gets a disproportionate benefit (a “hold-out premium”) for joining the pool compared to others already in. This is not distinctive to gene patents, but it could surface as a problem if patent pools begin to emerge.
The blocking effect is related to the somewhat different phenomenon of a “penumbra” effect. We characterize a penumbra as activities that are not strictly speaking infringing activities but that in practice result from controlling one or a few patents. This phenomenon appears in this case study because having rights to some common variants can in effect force those who want genetic testing to go to a particular single provider, even though no one can know in advance whether the mutations for which that provider has exclusive rights are actually the ones that cause symptoms in that individual.
One important purpose of seeking genetic testing for hearing loss is to identify the precise molecular cause of the symptoms. So if one testing service retains exclusive rights to test for a common variant, then everyone will of course need to test for that variant, and therefore will send samples to that service, even though the patient may actually have some other mutation—whether unpatented, discovered by someone else and patented, or that have not been discovered yet. By having rights to one common variant, therefore, a service can force all who seek genetic testing for an entire clinical syndrome to come to it, even if its intellectual property covers only a fraction of all possible mutations. The owner of the key patent thereby controls not only his or her own intellectual property but also collateral space. This enables the holder to accumulate knowledge and expand its intellectual property. All those seeking genetic testing will come to this service, and new mutations will thus be found by it, leading to more patents for mutations for that condition. By this mechanism, a monopoly on the original discovery is leveraged to future discoveries and future patents on new mutations that no one has discovered before, in the clinical penumbra of the originally patented test.
The penumbra effect in effect expands the intellectual property controlled by the initial patent holder, but it can also create some perverse incentives for subsequent inventions falling in the penumbra of the original patent. Those discovering a hearing loss mutation may think about simply leaving the discovery in the public domain. This might even be socially optimal by making the discovery available for both scientific progress and also making it easy for any testing service to incorporate the new discovery into ongoing testing. But if one service is controlling the testing because it has patent rights to common variants, then leaving the discovery unpatented merely fuels that service's advantage. The institution making the new discovery will thus face several choices: (1) patent and license to the dominant provider, getting a piece of the action (and thus increasing costs in general, both transaction costs of getting and licensing the patent, but also the pass-through costs to the provider and even higher pass-through costs to end-users—this is the option taken by many institutions in this case study); (2) patent and nonexclusively license; (3) do not patent and forego royalties (true for several in this case study); or (4) patent and license to an entirely different provider, setting up a mutual-blocking situation among service providers. To our knowledge, mutual blocking has not occurred in this case, but it did appear for long QT syndrome in a separate case study. All these options are socially suboptimal by one criterion or another (fairness, efficiency, or both). The phenomenon is one of the reasons that diagnostic licensing will be a difficult policy problem to solve.
Finally, when a clinical condition requires testing for mutations or uses methods covered by many patents, this can increase costs because of royalty stacking (because payments to many patent owners are required). This is a common problem in technology licensing and not distinctive to diagnostics. The solutions include having a cap on total royalties, clauses in licenses that permit royalty reduction if further licenses become necessary to practice an invention, patent pools, and renegotiation rules. These solutions are all dependent on licensing terms. Because licensing is largely opaque, those out-licensing or in-licensing technologies have no obligation to share terms of licensing with us. We do not know the extent to which these issues have been addressed in patent licenses that affect genetic testing for hearing loss.
In this case study, we assess the patent status of hearing loss genes and go as far as we can in judging whether or not they pose the potential for a patent thicket, or anticommons, and also the possibility of blocking patents and the penumbra effect. To our knowledge, royalty stacking was not identified as a major problem, although some have wondered about it in interviews. Our main findings are
-
Most hearing loss genes identified to date are not patented. It does not follow that testing for mutations in these genes is freely available, because of the penumbra effect.
-
Testing for Connexin 26 gene mutations, which account for up to half of all nonsyndromic recessive hearing loss cases, is patented.
-
Of the five most commonly tested hearing loss genes, three (GJB6, SLC26A4, and MTTS1) are not patented. Clinical testing is offered for each of these genes by several providers listed on the GeneTests.org website.
-
Testing for mutations in genes involved in less common forms of hearing loss is predominantly offered on a research basis, if it is available at all. Laboratories doing genetic testing for research purposes are generally not CLIA-certified.
-
The Institut Pasteur holds two patents (US 5,998,147 and 6,485,908) for the GJB2/Connexin 26 gene and for detecting its most common deletion mutation 35delG. GJB2 patents have been exclusively licensed, apparently with territory of use restrictions, to the for-profit company Athena Diagnostics for testing in the United States, Canada, and Japan. (The documents that specify terms of licensing, including territorial restrictions, are not public, so we can only infer such terms.)
-
Cedars-Sinai Medical Center holds a patent (US 5,506,101) that covers MTRNR1 mutation testing, specifically testing for the most common A1555G mutation. This patent is also exclusively licensed to Athena Diagnostics.
LESSONS LEARNED
Research
Research on both rare and common forms of hearing loss appears to have progressed independently of patenting status. There is no evidence that patents have had any positive or negative impact on hearing loss genetics research.
-
Research on microarray and chip-based diagnostics for hearing loss is being performed by multiple groups. These diagnostics include patented genes and mutations and are currently offered on a research-only basis in the United States.
-
Concerns about increased patent enforcement have been raised by some researchers, who worry about both research and clinical access.
Development and commercialization
-
We found no evidence that patents accelerated or inhibited hearing loss test development.
-
Diagnostic tests for both patented (GJB2, MTRNR1) and unpatented genes (SLC26A4, GBJ6, and MTTS1) have been developed and are offered as a clinical service by several providers. Demand for testing and the extent of research on hearing loss appear to be the primary factors that determine whether diagnostic testing for a particular hearing loss gene is offered as a clinical service at that institution.
-
Several providers offer testing panels that include both patented and unpatented tests, e.g., GJB2/Cx26 and GJB6/Cx 30 and MTRNR1 and MTTS1.
-
Testing for GJB2 mutations, which is licensed exclusively to Athena Diagnostics in the United States, was offered as early as 1998. At least 19 providers offered the test in the U.S in January 2009, a majority of which are academic medical centers. However, there have been intermittent enforcement efforts by Athena Diagnostics and some laboratories have stopped testing. In August 2008, one provider (Boston University's Center for Human Genetics) stopped offering Connexin 26 and MTRNR1 testing after Athena's enforcement actions. The recent discontinuation of ASRs offered by Third Wave Technologies to detect the 35delG mutation has increased concern about inability to circumvent patents covering 35delG mutation detection controlled by Athena. (Third Wave Technologies Inc. was acquired by HoloLogics Inc. in June 2008 and has discontinued marketing several ASRs for genetic testing, including Connexin 26 mutation testing, for business reasons.5) This may change the number of providers offering GJB2 testing. Laboratories previously using a two-tiered approach for GJB2 testing, first detecting the 35delG mutation with the ThirdWave InvaderTM assay, followed by full sequencing, especially if the sample is negative for 35delG mutations, may now be prevented from reporting out 35delG mutations. This may limit providers from performing clinically meaningful testing because 35delG is the most common GJB2 mutation and some providers may stop offering the test altogether, especially if Athena steps up enforcement activity.
-
The price of genetic tests for hearing loss does not appear to correlate with patent status alone. The most expensive test is for Pendred Syndrome, and involves full sequence analysis of SLC26A4. There are no patents associated with the SLC26A4 gene and average test price is ∼$1,700. In contrast, testing for GJB2, which is patented, has a list price ranging from $336 to $818. However, the price per amplicon for full sequence analysis of GJB2 ($140.80 – $430/per amplicon) appears to be higher than SLC26A4 sequencing prices, which range from $55.00 to $125.25/per amplicon. This price differential cannot be attributed to patents or licensing, however, because most providers of GJB2 testing probably do not have sublicenses from Athena. (Athena states it has not issued sublicenses.) Factors such as how labor and fixed costs are distributed in test pricing may contribute to this price difference.
-
The cost for GJB2 full sequence analysis offered by Athena Diagnostics ($575) is nearly $100 more than the average price of the same test offered by the other providers. Athena's price is nonetheless in the middle of the price range for full-sequence analysis offered by universities, hospitals, and academic medical centers ($290–$816). The price per amplicon for GJB2 sequence analysis offered by several nonprofit providers (range $140.80–$430) is comparable to Athena's price ($287.50).
-
The cost of the MTRNR1 test offered by Athena Diagnostics ($365) is higher than the price of the test offered by universities and hospitals ($150–$285, average price $210). Athena's higher price is not necessarily because of patents, however, and other factors may contribute to price difference.
-
Testing for the MTTS1 gene, which is not patented, is offered at prices comparable (average price $238) to MTRNR1 by universities and hospital-based providers. The test is not offered by any commercial testing providers, including Athena Diagnostics.
-
The SoundGeneTM diagnostic panel developed by Pediatrix includes testing for the most common mutations associated with hearing loss, including GJB2/Connexin 26. Athena Diagnostics has negotiated a sublicense with Pediatrix for Connexin 26 testing. A guaranteed royalty stream from high volume of testing associated with newborn screening follow-up was a likely motivator of this agreement.
Communication and marketing
-
Patents on hearing loss genes and related genetic tests appear to have little to no impact on dissemination of information about genetic testing or on how tests are marketed.
-
Athena Diagnostics does not engage in direct-to-consumer marketing. Athena markets primarily through a sales force keyed to clinical specialists. Athena does not have a sales force dedicated to the marketing of hearing loss tests to pediatricians or hearing loss specialists, rather its sales representatives address many neurological and neuromuscular conditions.
Adoption by clinical providers
-
To date, exclusive US licenses to patents on Connexin 26 and MTRNR1 testing do not appear to have secured Athena Diagnostics sole provider status. Although Athena Diagnostics is the reference provider, a number of additional providers, most of which are academic medical centers, are listed as providers of clinical testing at GeneTests.org. However, Athena has intermittently enforced its patents, and laboratories remain concerned about future enforcement activity.
-
Negative effects of patents and licensing practices on adoption of genetic tests for hearing loss by providers are not readily apparent, although concerns were expressed in interviews. As early as 1998, 10 providers offered GJB2 testing. The number of providers listed on GeneTests.org has risen to 18 since then. Nine providers for MTRNR1 testing are listed on GeneTests.org. However, there has been intermittent enforcement, and some providers have ceased offering some patented tests. We cannot determine how many laboratories decided against offering tests in the first place due to concerns about future patent enforcement.
-
Athena Diagnostics has sent at least three notification letters to other providers. In one instance, the UCLA Diagnostic Molecular Pathology Laboratory (nonprofit) stopped offering a test (GJB2 and GJB6 as part of the panel) and did not negotiate a sublicense, citing substantial up-front payment as an impediment. GeneDx (for-profit) continues to perform full sequence analysis for Connexin 26 to identify mutations associated with a rare skin condition, Keratitis Ichthyosis Deafness (KID), and agreed not to report hearing loss-associated mutations that are discovered during its full sequence analysis. In August 2008, the Center for Human Genetics of Boston University agreed to stop offering GJB2 testing, along with many other tests for which Athena Diagnostics holds an exclusive license.6
-
Providers of GJB2 and MTRNR1 testing presumably either collect samples and send them to Athena or offer the service without a sublicense from Athena Diagnostics. Athena states that no sublicenses for hearing loss testing have been negotiated with universities or academic medical centers to date.
Consumer utilization
-
We found no evidence that consumer utilization of these tests is impeded by patents.
-
A large number of providers offer these tests with a wide price range.
-
Athena Diagnostics does not engage in direct to consumer marketing. There is no evidence that tests may be over utilized by consumers.
-
Given the lack of clear correlation between the costs of these tests and patent or license status, there is no evidence that patenting or licensing has hindered consumer utilization in the United States because of test price.
-
Some consumers (such as those covered by MediCal) may not have tests such as Connexin 26 testing covered by their insurance or health plan, because the reference provider Athena Diagnostics does not have a contract with that program. Access for these consumers therefore depends on the availability of additional providers who may have contracts with Medicaid or entails direct out-of-pocket payment by consumers. Uncertainty surrounding whether these alternate providers will face enforcement or will stop testing creates an unstable situation.
Adoption by third party payers
-
In our informal phone survey, test providers indicated that genetic tests for hearing loss are usually covered by insurance.
-
Although comprehensive data on the coverage position of all major insurers for all hearing loss tests are not available, it is unlikely that patents have had significant impact on the adoption of tests. CIGNA HealthCare, for example, covers testing for GJB2 (patented) and GJB6 (unpatented).
CLINICAL AND SCIENTIFIC BACKGROUND
Hearing loss refers to the permanent, bilateral or unilateral, sensory or conductive, loss of hearing averaging 30 decibels or more in the frequency region important for speech recognition.3 Hearing loss can present at different stages in life, and therefore can be classified as prelingual (before learning to speak) or postlingual (after having learned a language). Prelingual hearing loss may be congenital or late-onset. Profound congenital hearing loss occurs in 1.8 per 1,000 live births in the United States. The prevalence increases to 2.7 per 1,000 among those below 5 years of age. During the teenage years, prevalence increases to 3.5 per 1,000.7 The lifetime societal costs for childhood hearing loss are estimated at $1.1 million per person, including lost productivity, special education, vocational rehabilitation, medical costs, and assistive devices attributable to deafness. Universal audiological newborn hearing screening programs have been introduced in the United States to reduce speech, social and emotional development problems experienced by children through early detection and intervention. At least 37 states have universal newborn hearing screening legislation and every state has early hearing detection and intervention (EHDI) programs, which screen approximately 93% of all infants.
As a heterogeneous trait, hearing loss has many environmental and genetic causes. Its incidence varies over time and across populations (Fig. 1).7 Environmental causes, such as infections, account for approximately half of hearing loss cases. Congenital cytomegalovirus (CMV) infection, in particular, is responsible for as much as 10% of congenital hearing loss.7 Genetic causes account for the other half of hearing loss cases. Hearing loss typically occurs due to abnormalities in single genes or sometimes gene pairs. A multitude of different genes and gene pairs (at least 65 genes and 110 choromosomal locations) have been implicated. Many others may yet be discovered.7
Genetic hearing loss can be further classified as “syndromic” and “nonsyndromic,” depending on whether it is associated with other clinical features (syndromic) or not (nonsyndromic).7 Syndromic cases represent about 30% of genetic hearing loss cases overall and encompass at least 400 syndromes and a similar number of genes. Nonsyndromic hearing loss or impairment (NSHL or NSHI) comprises the other 70% of genetic hearing loss cases and involves at least 100 loci, which can further be broken down by pattern of inheritance. NSHL loci include 55 autosomal recessive, 41 autosomal dominant, four X-linked, and two mitochondrial loci. Different mutations at the same locus (chromosomal location, usually a gene) can present as either nonsyndromic or syndromic hearing loss.3 Mutations in different genes may also result in the similar phenotypes (clinical symptoms and signs).7 A listing of nonsyndromic and syndromic hearing loss disorders and loci, including genes, genetic tests, and associated patents, is presented in the appendix (Appendix 1 and 2).
FIVE MOST COMMON GENETIC TESTS FOR HEARING LOSS
Given the numerous hearing loss genes, we have chosen to focus on the five genes that are most commonly tested for: GJB2/Connexin 26, GJB6/Connexin 30, SLC26A4/PDS, MTRNR1, and MTTS1 (M. Watson, American College of Medical Genetics, personal communication, 2007).
GJB2
Mutations in GJB2, or Gap Junction Protein Beta-2, have by far the highest frequency among genetic causes of deafness and hearing loss, accounting for up to 50% of cases of profound deafness caused by DNA mutations (Table 1).3 GJB2 encodes Connexin 26 (Cx26), a hexameric gap junction protein widely expressed in the cells and tissues of the cochlea.7 The link between GJB2 and nonsyndromic deafness at the DFNB1 locus was first published in a 1997 Nature article by Kelsell et al. 8 at St. Bartholomew's and the Royal London School of Medicine and Dentistry, Queen Mary and Westfield College. (NSHL loci are classified “DFNB” for recessive, “DFNA” for dominant, “DFN” for X-linked, and “DFNM” if they modify the expression of other genetic forms. The loci within each class are then numbered.7) That same year at the Institut Pasteur, Petit et al. discovered the most prevalent GJB2 mutation, 35delG.9 The Institut Pasteur holds two patents (US 5,998,147 and 6,485,908) for the GJB2/Connexin 26 gene and detection of its common deletion mutation. Patent applications were filed in August 1997 and granted in 1999 and 2002. The Institut Pasteur also holds patents for Connexin 26 in Canada and Japan. We have found no granted patents in Europe, although applications appear to have been filed. Patents have been exclusively licensed to Athena Diagnostics, and we infer these were licensed for use in the U.S., Canada, and Japan. In Europe, the exclusive license for Connexin 26 testing went to Nanogen, a provider of molecular diagnostic services.10 As of February 2008, “Molecular Diagnostics for Prelingual Hearing” was still listed as a diagnostic technology available for licensing at the Institut Pasteur technology transfer website. This suggests either that existing licenses to Nanogen and Athena do not exhaust all territories worldwide or that provisions for particular fields of use have been retained by Institut Pasteur. As of January 2009, the technology is listed under Genomics (ID 98.30); however, it is unclear if the technology listed relates to testing for GJB2 specifically. Previous versions of the site accessed in February 2008 indicated that the technology listed was GJB2 testing.11 We have no direct information about whether Institut Pasteur has granted any additional licenses in Europe or the United States. The Institut Pasteur was contacted by e-mail to clarify the status of licenses but did not respond.
Based on data gathered through our telephone survey of providers (identified through GeneTests.org), testing for GJB2/Connexin 26 in the United States began as early as 1998. Kenneson et al. 12 surveyed Connexin 26 testing providers in the United States in 1999 and 2000 (10 eligible providers in 1999 and 8 providers in 2000). Based on provider information at GeneTests.org, 19 U.S. providers (18 nonprofit and 1 for-profit) offered full sequence analysis, which is the most common type of GJB2 testing. PCR-based sequence analysis has been facilitated by the relatively small size of the single GJB2 coding exon.13 Full sequence analysis is appropriate given that more than 195 GJB2 mutations have been identified,7,14 which vary in frequency by race/ethnicity and family history.13 The average price of the GJB2 full sequence test among nonprofit providers is $472.35 compared with the list prices of $575 quoted by Athena Diagnostics, the reference provider (Table 1). On February 10, 2010, Athena Diagnostics told the Secretary's Advisory Committee on Genetics, Health, and Society, “Athena charges $340-575 for GJB2 testing” (T. Fenton, Thermo Fisher Scientific, which owns Athena Diagnostics, personal communication, 2010).
Prices for full sequence analysis of Connexin 26, when normalized for number of amplicons, are also quite variable among providers. The unit price for the test offered by Athena Diagnostics is in the middle of the price range of nonprofit providers (Table 2). The average price per amplicon of tests offered by nonprofit providers is ∼$231 and is comparable to Athena's unit price for full-sequence analysis ($287.50). Although diagnostic billing codes provide some standardization for full-sequence tests, techniques and procedures are not identical among laboratories. The same billing codes are not always used, and the laboratories surveyed also likely have different overhead costs.
Although it appears that the number of U.S. providers offering Connexin 26 testing has increased to 19 from the 10 identified by Kenneson et al. 12 in 2000 (19 providers listed on Genetests.org offered full sequence analysis in January 2009), it is unclear whether the Institut Pasteur's exclusive license to Athena Diagnostics for Connexin 26 testing has deterred other laboratories from testing. Some listed services may send samples to Athena or to offshore providers. To date, it appears that Athena Diagnostics has not granted sublicenses to any other providers listed on GeneTests.org (M. Henry, Athena Diagnostics, personal communication, 2007). It is also not clear that patents and exclusive licensing have contributed to a pricing differential or monopoly pricing by a sole provider. The 14 nonprofit institutions we surveyed offer the test at varying prices, some comparable to the price of Athena Diagnostics, as shown in Tables 1 and 2.
GJB6
A significant portion (30–50%) of nonsyndromic genetic hearing loss is attributed to mutations in GJB6, or Gap Junction Protein Beta-6. Like GJB2, GJB6 is expressed in the cochlea and contributes to DFNB1 hearing loss. The GJB6 gene encodes Connexin 30 (Cx 30), a heteromeric gap junction protein that can form channels with Connexin 26, resulting in cases of digenic transmission (that is, the condition results from two different affected genes).7 The link between the >300kb GJB6 deletion and nonsyndromic DFNB1 hearing loss was first published in January 2002 in the New England Journal of Medicine by Ignacio del Castillo et al15 at the Unidad de Genética Molecular, Hospital Ramón y Cajal, Madrid, Spain. Genetic testing for GJB6 deletions in patients with hearing loss is linked to the genetic diagnosis of GJB2. GJB6 deletions are found in trans (that is, the genes are located on different chromosomes, suggesting the effect is mediated by a protein produced by the genes, rather than regulation of the genes themselves) with a mutant GJB2 allele and contribute to the same subtype of genetic deafness, DFNB1. The joint contribution of mutations in these two genes to nonsyndromic recessive hearing loss is about 30–50%. Although prevalence varies across populations, one North American study found a 2.57% prevalence of GJB2/GJB6 digenic cases among deaf individuals, with more severe hearing loss than is typical for GJB2 alone.13 However, a more recent study by Putcha et al. reported that the frequency of a >300Kb deletion in individuals bearing compound GJB2 and GJB6 mutations was only 1% in a large North American cohort. Data of Putcha et al. 16 suggest that this mutation may be quite rare. No U.S. patents or applications associated with the Connexin 30 gene or mutation testing were identified in our patent searches. Dr. Ignacio del Castillo, who first reported the GJB6 deletion mutation, confirmed that he had not applied for patents (I. del Castillo, Unidad Genética Molecular, Hospital Ramón y Cajal, personal communication, 2007). To date, seven (six nonprofit and one for-profit) providers offer Connexin 30 deletion analysis in the United States. The test appears to have been offered in the United States as early as 2002, based on our telephone survey of providers listed on GeneTests.org. The list price for GJB6/Connexin 30 testing averages $300 at nonprofit institutions and is $295 at the one for-profit laboratory.
SLC26A4
In 1997, Eric Green and colleagues at the National Human Genome Research Institute identified the SLC26A4, or PDS gene, which encodes the protein pendrin, a transporter of chloride, bicarbonate, and iodide.17 Mutations in SLC26A4 are implicated in a form of syndromic deafness (Pendred syndrome), as well as a form of nonysndromic deafness DFNB4. Pendred syndrome is the most common form of syndromic deafness and accounts for up to 10% of deafness. Pendred syndrome has an incidence of 7.15–10 per 100,000 births.17
Although both Pendred syndrome and DFNB4 involve severe hearing loss and an enlarged vestibular aqueduct, Pendred syndrome is also associated with thyroid goiter. In the absence of a goiter, Pendred syndrome is classified by an abnormal perchlorate discharge test.18 The severity of goiter is variable, and thyroid symptoms may not occur until late childhood or even adolescence. Pendred syndrome typically has a prelingual age of onset (before the critical period for language development), whereas nonsyndromic DFNB4-associated deafness tends to be postlingual.7 No U.S. patents relating to SLC26A4 were identified.
Based on our informal phone survey of providers, testing for SLC26A4 has been available since at least 2002. The most commonly offered test, full-sequence analysis, can detect disease-causing mutations in about half of multiplex and one-fifth of simplex cases.18 All six U.S. providers of full sequence analysis SLC26A4 testing are nonprofit institutions, and the average price is $1,686. The relatively high price of SLC26A4 full sequence analysis cannot be attributed to the existence of a patent or exclusive licensing. Rather, it appears that the cost of full sequence analysis relates to SLC26A4 being a large gene (∼77 Kb) with 21 exons encoding a 4.93 Kb transcript. Therefore, testing requires testing methods comparable in complexity and price to testing for inherited susceptibility to colon and breast cancer.7,19 The price/per amplicon for sequencing the SLC26A4 gene ranges from $55.00 to $125.25 when standardized for the number of PCR amplifications reactions performed. The number of amplicons for SLC26A4 gene sequencing is 20, which is the number of nucleic acid sequences targeted for amplification (based on the number of times CPT billing code 83898 is used by the provider). Four providers offer SLC26A4 analysis for specific mutations at lower costs ($635) than the full sequence analysis. Targeted mutation analysis has a sensitivity of 70% for heterozygotes and 91% for those homozygous for a mutation.7
MTRNR1 and MTTS1
Mitochondrial forms of moderate to profound NSHL result from mutations in either the MTRNR1 or MTTS1 genes in mitochondrial DNA, each of which accounts for fewer than 1% of hearing loss cases. MTRNR1 encodes 12S ribosomal RNA (12S rRNA), whereas MTTS1 encodes transfer RNA for serine (tRNA Ser[UCN]).20 The most common MTRNR1 mutation, A1555G, occurs with a 0.3% frequency in the United States.20 Prezant et al., from Cedars-Sinai Medical Center in Los Angeles, California, first reported the association between A1555G mutations and aminoglycoside-induced and nonsyndromic deafness in Nature Genetics in July 1993.21,22
MTRNR1 mutations may contribute to permanent, nonprogressive hearing loss either through: (1) susceptibility to aminoglycoside (antibiotic) ototoxicity, irrespective of dose, or (2) late onset hearing loss in the absence of aminoglycoside exposure. MTTS1-related hearing loss, in contrast, has a characteristic progression first appearing during childhood and with penetrance that varies by individual mutational load (more numerous mutations accompany earlier onset and more severe deafness).20 Higher mutation loads of some MTTS1 mutations also correlate with the manifestation of other clinical signs, such as palmoplantar keratoderma, or ataxia and myoclonus. The association between mutations in MTTS1 (tRNA–Ser [UCN]) and sensorineural deafness was first reported in 1994 by Reid et al. 23 at the University of Glasgow in Scotland, UK.
Cedars-Sinai Medical Center holds a patent (US 5,506,101) that covers MTRNR1 mutation testing, specifically testing for the A1555G mutation. The patent application was filed in June 1993 and granted in April 1996. Athena Diagnostics acquired an exclusive license for mutation testing for MTRNR1 from Cedars-Sinai Medical Center. Cedars-Sinai Medical Center also holds patents in Japan and Canada for MTRNR1 A1555G mutation and testing. No patents were filed in Europe. Our searches found no patents covering the MTTS1 sequence or genetic testing for its mutations.
MTRNR1 testing first became available in the U.S in 2000. Targeted mutational analysis is now offered by 10 U.S. providers. The two for-profit providers average a higher list price ($355) than the six nonprofit (university hospitals and medical center based) providers (average $210) (Table 1). Information about sublicenses from Athena Diagnostics for MTRNR1 mutation testing is not publicly available. In contrast, MTTS1 targeted mutation analysis has been available since 2004 and is offered by four nonprofit providers for an average price of $238 (Table 1). In addition, a subset of nonprofit providers also offers testing for a panel of mitochondrial mutations, including both MTRNR1 and MTTS1, for an average price of $438.
NEWBORN HEARING SCREENING
Because of the potential for language, social, emotional, and other developmental consequences in children whose hearing loss is detected after 6 months of age, a 1993 National Institutes of Health Consensus Development Conference endorsed universal newborn hearing screening.24 In 1999, the Health Resources and Services Administration and Centers for Disease Control and Prevention began funding state EHDI programs.25 At least 37 states have legislation for universal newborn screening for hearing. Today, EHDI programs exist in every state, providing screening for approximately 93% of all infants.26 The goals of EHDI programs are 3-fold: (1) to screen all newborns before 1 month; (2) to diagnose newborns before 3 months; and (3) to coordinate intervention before 6 months (see Appendix 3 for detailed flowchart).25 EHDI programs have reduced the average age for confirming hearing loss from 20 to 30 months (before the program), to 2 to 3 months (after implementation).
The EHDI programs miss some hearing loss cases, however, because prelingual hearing loss does not always present during infancy. SLC26A4 and A1555G-related hearing loss can appear after infancy, for example. Some cases of GJB2 deafness cannot be detected at birth. With an estimated nonpenetrance rate of 3.8%,27 EHDI programs are seen by some as an opportunity for more genetic testing as part of the evaluation process.28,29 Practical obstacles remain in screening programs for hearing loss, including uncertainty about the appropriate timing and role of genetic testing in the EHDI process.30 Survey data show that 20% of professionals who administer EHDI programs lack genetics training, which fuels concern about ordering and interpreting complex genetic tests.1
CLINICAL GUIDELINES FOR GENETIC TESTING
In 2002, the ACMG published clinical guidelines that incorporate genetic testing into the diagnosis of congenital hearing loss.3 The Cincinnati Children's Hospital Medical Center's testing paradigm exemplifies how hearing loss genetic test providers approach genetic evaluation (Appendix 4). A pretest session to explain the causes and types of deafness, along with testing options and modes of inheritance, is important. After the pretest session, the next step entails getting a family history and an individual patient history and conducting a physical examination to determine whether or not a diagnosis is apparent. If syndromic hearing loss is suspected, the ACMG recommends gene-specific mutation tests. The diagnosis of nonsyndromic cases is more complex, and relies on details of family history and individual symptoms. Individuals with hearing-impaired first-degree relatives, or two deaf parents, are also candidates for GJB2 testing. As the most common genetic cause of hearing loss, GJB2 is the first in a series of recommended tests.
If a GJB2 test reveals that an individual is a heterozygote, Cincinnati Children's conducts a follow-up GJB6 deletion screen. If the GJB2 test is negative, the ACMG calls for nonsyndromic mitochondrial testing, specifically for the A1555G and A7445G mutations. Cincinnati Children's distinguishes among the types of mitochondrial testing, suggesting MTRNR1 testing only in the presence of aminoglycoside exposure, and a full mitochondrial panel otherwise. After these initial rounds of genetic testing for GJB2 and mitochondrial mutations, the ACMG recommends post-test counseling and education. Given that 10% of deaf infants have culturally deaf parents, the availability of interpreters and the culturally sensitive interpretation of hearing loss test results are critical.3
After parents are informed of their options, follow-up and additional genetic testing may be recommended. Imaging studies may be ordered to consider the possibility of DFNB4 or Pendred syndrome, particularly for progressive hearing loss. Such imaging studies may include temporal bone imaging, to look for an enlarged vestibular aqueduct and/or cochlear dysplasia. If imaging studies have positive findings, mutation screening of SLC26A4 would be recommended.
Clinical utility of genetic testing for hearing loss
Genetic tests offer several advantages over conventional hearing loss evaluation without genetic testing. The benefits anticipated from genetic testing include29–31:
-
Reduction of additional time consuming, invasive, and expensive testing;
-
Choice of early interventions such as hearing aids, cochlear implants, or sign language that significantly improve language ability and quality of life outcomes;
-
Information on the progression of the condition;
-
Ability to monitor associated clinical manifestations and complications, particularly for certain syndromic forms of hearing loss;
-
Information on the chance of recurrence in the family that can inform reproductive decisions; and
-
Information pertinent to risks and health care decisions (e.g., avoiding aminoglycoside antibiotics among those with MTRNR1 mutations).
Genetic testing may be more sensitive and specific than traditional evaluation. A study at Cincinnati Children's Hospital found that 80% of hearing loss patients remained undiagnosed after traditional evaluation. Furthermore, genetic tests may facilitate earlier detection of hearing loss. Despite widespread newborn screening for hearing loss, a recent analysis showed that newborn screening can fail to identify some infants with two GJB2 mutations.13 The age at which the hearing loss was identified ranged from 12 to 60 months. A delay in detecting hearing loss has important implications for language acquisition and limits subsequent choices among management strategies. A study about cochlear implants reports, “There seems to be a substantial benefit for both speech and vocabulary outcomes when children receive their implant before the age of 2.5 years.”32 A white paper addressing the societal costs of hearing loss concludes that “early identification of deafness or hearing loss is critical in preventing or ameliorating language delay or disorder in children who are deaf or hard of hearing and allows for appropriate intervention or rehabilitation. Early identification and intervention have lifelong implications for language development.”33 The present value of lifetime societal costs for prelingual hearing loss is estimated as $1.1 million, which includes lost productivity, special education, vocational rehabilitation, medical costs, and assistive devices attributable to deafness.34
Cost effectiveness of genetic testing for hearing loss
We found no comprehensive cost effectiveness analyses of genetic testing for hearing loss. GJB2 testing may preclude the need for more expensive or invasive tests and provide the emotional benefit of knowing the cause as well as the clinical benefit of predictive information about progression and treatment options.28 A recent study at the Cincinnati Children's Hospital Medical Center demonstrated that when compared with simultaneous testing, which comprises a battery of tests including standard laboratory work-up, or diagnostic evaluation by imaging, a diagnostic algorithm with GJB2 genetic testing as the first step resulted in a possible savings of “$20,180 in imaging costs and $34,000 in laboratory test costs per 100 children” screened.35 The data on test-specific savings are in Table 3.35
Another study at the Children's Hospital of Alabama assessed the cost of a battery of laboratory tests to evaluate hearing loss, including thyroid function, congenital infection, electrocardiograms, urine analysis, and serum phytanic acid levels, weighed in at more than $1,300, compared with the one-time $425 cost of a GJB2 genetic test.31
Although the benefits of GJB2 testing have yet to be quantified, researchers note the ability of GJB2 tests to define chance of recurrence, i.e., if a child is GJB2 positive, a hearing couple knows that there is a 25% chance they will have a deaf child in each future pregnancy, and a deaf couple (each with GJB2 deafness) can learn that there is a 100% chance they will have deaf children.31 GJB2 testing may also be important given the success of cochlear implants among GJB2 positive individuals. A GJB2-positive individual may develop the same speech skills as an individual with normal hearing if the hearing loss is diagnosed and the cochlear implants are prescribed at a young enough age.31
In the case of nonsyndromic mitochondrial testing, quantitative data are scarce. The benefits, however, are significant, considering that a positive A1555G test could prevent an infant from being exposed to aminoglycoside antibiotics, thereby preventing hearing loss. Another consideration associated with testing for these mutations is that aminoglycosides are often given before genetic testing has been performed because the infectious process has to be treated without delay. So, in reality, the test is only beneficial if conducted before the onset of infection, or if test results can be turned around within a few hours. Because of increased numbers of premature births and widespread use of gentamycin in neonatal intensive care units, neonatologists have been particularly concerned about A1555G mutations and aminoglycoside exposure. However, in the absence of point-of-care testing, it would require screening parents before delivery or testing newborns to identify those at high risk of hearing loss from aminoglycoside use. For an individual with an A1555G substitution and no exposure to aminoglycosides, the probability of developing hearing loss by age 30 drops from 100 to 40%.13 Given the lifetime cost associated with prelingual hearing loss of $1.1 million, that amount could be averted by each case of deafness avoided. Because aminoglycosides are only prescribed in the event of severe in-hospital infections, the number of individuals prescribed aminoglycosides and estimates of the increased risk of untreated infection would have to be factored into any cost-effectiveness calculation.36
The limitations of genetic testing for hearing loss also have to be taken into account in cost effectiveness analysis. Because genetic deafness is population- and ethnicity-specific, relative frequencies should first be refined to best represent the population being studied. Although GJB2 testing may confer large benefits for individuals who test positive, those benefits also have to be measured against the costs for individuals who test negative. Individuals who test negative for GJB2 mutations may have to undergo additional medical and/or genetic testing or may experience emotional difficulty when attempting to comprehend the meaning of the confusing and inconclusive test results.31
Molecular testing for hearing loss: new developments
If recommendations to include genetic testing as part of expanded EHDI programs and clinical follow up of infants identified by universal newborn hearing loss screening are followed, then the volume of genetic testing for hearing loss could rise dramatically.29,37 Testing for mutations associated with the most common forms of syndromic and NSHL plus congenital CMV infection can determine the cause of hearing loss in most cases of congenital hearing loss. Preciado et al. 35 conclude that introduction of genetic testing (specifically GJB2 testing) for hearing loss in the clinical evaluation paradigm is cost effective.
Recently, Pediatrix introduced genetic testing services for hearing loss. Pediatrix is one of the largest providers of newborn metabolic screening and newborn hearing loss screening services in the U.S. Pediatrix's SoundGeneTM Screening panel includes mutations associated with the most common forms of nonsyndromic and syndromic hearing loss. It also includes testing for common mutations in the mitochondrial MTTS1 gene, as well as testing for CMV infection (determined by measurement of copies of viral DNA, and therefore also, in essence, another genetic test). CMV infections account for up to 25% of congenital hearing loss caused by pathogenic agents. The SoundGeneTM panel includes:
The SoundGene™ Screening Panel38
Connexin 26 (Cx26) GJB2 mutations
35delG 167delT
235delC M34T
Connexin 30 (Cx30) GJB6 large deletion
309 kb large deletion
Mitochondrial mutations
7445A>C (A7445C) 961T>C (T961C)
7445A>G (A7445G) 961T>G (T961G)
7444G>A (G7444A) 961 delT + C(n)ins
Pendred SLC26A4 mutations
L236P 1001 + 1G>A
E384G T416P
CMV DNA
The SoundGeneTM Screening Panel was introduced in December 2006. The list price is $95.00 (Pediatrix, personal communication). A U.S. patent application for the SoundGene™ Screening Panel is pending (Application U.S. 20040038266A filed in 2003, see Appendix 5). SoundGene™ has also been trademarked. The test is described as a “quick and cost-effective alternative” and has an average turnaround time of 48 hours. Genetic counseling services for interpretation of test results and consultation are available through Pediatrix. Pediatrix has acquired a sublicense from Athena Diagnostics for testing of the Connexin 26 35delG mutation, which is included in the SoundGeneTM panel. Pediatrix is the only provider to which Athena reports having issued a sublicense for Connexin 26 testing in the United States. Although we do not have details of the licensing agreement and royalties, it is likely that the anticipation of high testing volume by Pediatrix as part of its newborn hearing loss screening services was an incentive for this agreement. Interestingly, however, the SoundGeneTM panel does not include testing for the common A1555G mutation in the mitochondrial MTRNR1 gene (Patent no: US 5,506,101) that is also exclusively licensed to Athena Diagnostics.
High-throughput molecular diagnostics for hearing loss
With over 90% of newborns currently being screened for hearing loss and the potential for expanded EHDI programs to include molecular screening, genetic testing may shift to newer platform technologies for high-throughput genetic testing. Microarray-based genetic testing is being actively pursued as an efficient, reliable, and potentially cost-effective tool when many mutations in a gene or numerous different genes must be tested. Hearing loss could be such a case. Because hundreds of loci are involved in the biology of hearing loss and additional genes and mutations may yet be discovered, microarray chips that can readily add new genes or mutations might help address both research and clinical needs. Microarray-based diagnostic testing for hearing loss might make it more flexible, less expensive, and more comprehensive while being as sensitive and specific as existing genetic tests.
Several groups report working on microarray-based diagnostic testing for hearing loss. Henrik Dahl and coworkers from the University of Melbourne and Children's Royal Hospital in Australia have developed a hearing loss microarray that detects 15 common mutations in the Connexin 26/GJB2, SLC26A4, USH2A genes and mitochondrial 12S rRNA.39 This array-based chip was validated using DNA from 250 patients diagnosed with sensorineural hearing loss. It detected the mutations for which it was designed with 100% accuracy, and Siemering et al.39 report that no false positives or negatives were detected. Commercial development of the hearing loss biochip is suggested by U.S. patent application US20070009887A1, “Genotyping of deafness by oligonucleotide microarray analysis,” which was filed in November 2003, listing Victoria Siemering and Henrik Dahl as the inventors (Appendix 5).
Another microarray diagnostic chip was recently reported by Schrijver and coworkers in September 2006.40 Their diagnostic panel includes 198 mutations in eight genes most commonly associated with nonsyndromic sensorineural hearing loss. A patent application US20070134691A1 for this diagnostic has been filed by Schrijver and coworkers (Appendix 5). The chip uses arrayed primer extension technology, first developed by Shumaker and Caskey (Baylor College of Medicine, Houston Texas) and Metspalu and coworkers (University of Tartu, Estonia).41,42 Patents covering this technology, US 6,153,379 and US 7,001,722, were granted in 2000 and 2006.
The hearing loss chip tests for mutations in Connexin 26/GJB2, Connexin 30/GJB6, GJB3, GJA1, SLC26A4, SLC26A5, and mitochondrial 12S rRNA and tRNA Ser[UCN] and includes the commonly tested Connexin 26 35delG and A1555G MTRNR1 mutations, both of which are licensed exclusively to Athena Diagnostics. Currently, this diagnostic assay is being offered on a “research only” basis at the Molecular Pathology Laboratory at Stanford University by Schrijver and coworkers (I. Schrijver, Standford University, personal communication, 2007). Genetic testing for hearing loss using this diagnostic chip is being offered by Asper Biotech.43 Asper Biotech, located in Tartu, Estonia, was founded in 1999 with Dr. Andres Metspalu as its scientific advisor, and has expertise in developing and validating highly customized single nucleotide polymorphism/mutation screening assays. Asper Biotech also offers genetic testing services for diseases including cystic fibrosis, Usher Syndrome, retinitis pigmentosa, thalassemia, and a panel of genetic disorders common in the Ashkenazi Jewish population.44 Dr. Andres Metspalu at University of Tartu, Estonia, confirmed that the testing services offered by Asper Biotech are for research. The hearing loss test and other genetic tests offered by Asper Biotech are used by some academic medical centers and hospitals in the U.S in clinical research studies, often as part of collaborative projects (A. Metspalu, University of Tartu, personal communication, 2008). It is not clear that any licenses have been negotiated by Asper Biotech with Institut Pasteur or Nanogen for the use of Connexin 26 mutation testing or with Cedars Sinai Medical Center for MTRNR1 mutation testing, but a license might not be required because they are not patented in Estonia. (Patent applications covering Connexin 26 and MTRNR1 mutations and diagnostic testing were never filed in Estonia.) Dr Metspalu confirmed that there is no patent protection for Connexin 26 and MTRNR1 mutations and testing in Estonia. However, he indicated that if Asper Biotech did decide to market the hearing loss test in the United States, it would have to acquire sublicenses for all the relevant intellectual property and would have to factor royalty payments into its business plan. (We are not sure we concur with this judgment if the test itself were conducted in Estonia.)
Additional groups in the United States (shown in Appendix 5) are exploring the use of kits and microarray diagnostics for high-throughput, comprehensive, and cost-effective molecular screening. Dr. John Greinwald and colleagues at the Cincinnati Children's Hospital previously reported that a diagnostic paradigm incorporating genetic testing during clinical evaluation of hearing loss proved more cost effective than standard simultaneous laboratory work-up.35,45 Dr. Greinwald's group is now testing a microarray-based diagnostic gene chip that includes 13 genes associated with hearing loss. This collaborative project between Cincinnati Children's Hospital Medical Center and the University of Cincinnati Medical Center is being performed at the Computational Medicine Center and is in an early phase of integrity and validation studies.46 Dr. Greinwald and colleagues have also filed U.S. patent applications US20050112598A1 and US20040166495A1, “Microarray-based diagnosis of pediatric hearing impairment-construction of a deafness gene chip,” based on the development of this gene chip (Appendix 5). In a recent article, Li et al.47 reported using a multiplex allele-specific PCR-based universal array, which combines Amplification Refractory Mutation System with array technology for clinical diagnostic testing of hearing loss mutations in parallel.
Several groups have thus developed high-throughput diagnostic testing for hearing loss. U.S. patent applications filed by at least two of these groups on microarray-based gene chips suggest the potential for future commercialization of these diagnostic tests. However, we do not know if these tests will be adopted by clinical providers. Factors including test sensitivity, clinical utility, and cost of the test are likely to significantly affect their uptake.
We also do not know whether the chip makers and testing service providers have licensed patents for mutations and methods associated with genetic tests for hearing loss. Neither have we studied whether use of short DNA probes on these chips would infringe existing patents, as this would require detailed analysis of claims and deep knowledge of the testing methods.
Finally, we note that full-genome sequencing technologies are progressing apace, and if such analysis became possible, then the basis for genetic testing would be individual genomic sequencing and comparing that sequence to known mutations associated with all genetic forms of hearing loss, rather than tests specifically keyed to hearing loss. The intellectual property implications are unclear, as they are for genetic testing of other clinical conditions.
THE IMPACT OF PATENTS ON ACCESS TO HEARING LOSS TESTING
Research
We found no evidence about positive or negative effects of hearing loss gene patents on research in the field of hearing loss genetics. Basic research to determine the associations between candidate genes and their roles in various forms of hereditary hearing loss has steadily progressed. Research appears to be proceeding rapidly on rare forms of deafness that offer the prospect of a small market for diagnostic testing and are therefore unlikely to provide significant monetary incentives for genetic testing. Most genes associated with different forms of syndromic and nonsyndromic deafness are not patented (Appendix 1 and 2). Even among the five most commonly tested hearing loss genes, which are presumably of greatest commercial interest, three genes are not patented. It is unclear whether patents or the potential for commercialization provided an incentive for the research. At least two research groups at nonprofit institutions were engaged in studies to identify Connexin 26 gene mutations. Publications reporting the identification of mutations in Connexin 26 by Kelsell at al.8 (Queen Mary and Westfield College, UK) and Christine Petit et al.9 (Institut Pasteur) were submitted in January (published in May) and August (published in November) of 1997 to Nature and Human Molecular Genetics, respectively. Although the UK group does not appear to have applied for a patent, Christine Petit and Institut Pasteur secured US patents on GJB2/Connexin 26 and its mutations in December 1999. Petit and colleagues applied for a patent in August 1997, the same month they submitted their findings for publication. Dr. Fischel-Ghodsian at Cedars-Sinai Medical Center submitted their report on the MTRNR1 A1555G mutation to Nature Genetics in February 1993 (published July 1993).21 The corresponding patent application on detection of A1555G mutation was filed on June 30, 1993, 4 months after submitting for publication, and granted to Cedars-Sinai in April 1996. Although these chronologies suggest that scientific publication and patenting activities proceeded in parallel, we cannot determine if journal submissions were in fact delayed in the first place to prepare patent applications for parallel filing.
Without information on the royalties Institut Pasteur and Cedars-Sinai Medical Center receive from the licenses to Athena Diagnostics for Connexin 26 and MTRNR1 testing, it is also difficult to comment on the impact that these patents have had on supporting subsequent basic research at these institutions. Such support would be one of the positive effects of patents.
A substantial amount of clinical research has been performed, for example on the prevalence of Connexin 26 mutations in different populations, and on new methods for diagnostic testing including array-based diagnostics. Such studies include genetic testing for mutations covered by patents and licensed exclusively to Athena Diagnostics (Connexin 26, MTRNR1). However, researchers at academic medical centers told the authors that they remain concerned about the consequences of future enforcement activity by Athena Diagnostics on the clinical testing and clinical research. Researchers warn that uncertainty about whether an academic medical center or reference laboratory may be required to stop testing and the absence of a clearly stated policy about research use from Athena Diagnostics may have chilling effects on clinical research.
Development and commercialization
Genetic tests for Connexin 26 and MTRNR1 which are patented, and for GJB6, SLC24A6, and MTTS1, which are not covered by patents, have been developed and are offered by several providers at similar prices. Several providers have in fact developed test panels that include both the patented Connexin 26/MTRNR1 as well as the unpatented Connexin 30/MTTS1 tests. The acquisition of an exclusive license for Connexin 26 diagnostic testing in the US was presumably integral to Athena Diagnostics' plan to commercialize these tests. GJB2 testing was offered by at least 9 providers in the United States as early as 1998. The number of providers listed at GeneTests. org has doubled since 1999–2000.12 Testing for the patented genes GJB2 and MTRNR1 and their most common mutations is offered by more U.S. providers than testing for the unpatented genes SLC26A4, GJB6, and MTTS1. This is not entirely surprising given that GJB2 mutations account for up to 50% of cases of NSHL. The majority of laboratories listing the tests are academic health centers.
Clinical testing for MTRNR1 in the United States may have been delayed. The association of MTRNR1 mitochondrial mutations to hearing loss was published as early as 1993, yet clinical testing appears to have become available only in 2000. In our telephone survey, many laboratories were unable to provide data on when they first made this test available. A more systematic and detailed survey of providers might help determine if patents impeded or deterred providers from developing these tests, because we did not query providers specifically about this issue.
It is difficult to assess exactly how much of a price premium the exclusive license provides Athena Diagnostics, or what impact the patent licenses have on volume. According to Athena Diagnostics, to date only one sublicense for Connexin 26 testing has been granted (to Pediatrix). Thus, the list price of the other providers must not include royalty or licensing fees. The price range can be attributed to factors such as overhead costs at different institutions. In the case of testing for MTRNR1, the price offered by both for-profit providers is on average $145 more than the price of the test provided by nonprofit institutions. The $365 list price of the test offered by Athena Diagnostics is nearly 73% higher than the average list price offered by other university and hospital-based providers. In contrast, testing for the unpatented MTTS1 gene is offered by only four nonprofit providers and at prices comparable to MTRNR1 testing services offered by these providers. MTTS1 testing is not offered by Athena Diagnostics.
Costs of hearing loss tests do not appear to correlate strongly with patent status. For instance, the price of the most expensive test–SLC26A4 full sequence analysis–can be attributed mostly to the costs of sequencing a large gene. The relatively high cost of the SLC26A4 testing also affects fewer consumers, because Pendred's syndrome accounts for a small fraction of hearing loss cases and testing is recommended only to follow up on positive imaging findings.
Communication/marketing
It appears that patents on DNA sequences and methods for hearing loss genetic testing have had little impact on the dissemination of information about such tests or how they are marketed. We found no evidence of direct-to-consumer marketing. In the course of a phone conversation, Dr. Michael Henry, Vice President of Business Development at Athena Diagnostics, clearly stated the company's commitment to refrain from direct-to-consumer marketing and emphasized that Athena relies primarily on physician-prescribed testing. He also indicated that while Athena Diagnostics does have sales representatives who communicate information about genetic testing for neurological conditions to neurologists and medical practices, there is no sales force specifically committed to marketing hearing loss genetic testing to pediatricians and specialists (e.g., otolaryngologists and audiologists).
Adoption by clinical providers and testing laboratories
Any effects of patents on adoption of hearing impairment genetic tests by clinical providers are not readily apparent.
The exclusive license procured by Athena Diagnostics for Connexin 26 and MTRNR1 testing does not appear to have established Athena Diagnostics as the sole provider. However, the number of providers currently available may not fully capture the effects of patents on provider adoption. According to Dr. Michael Watson, Director of the ACMG, “Athena aggressively enforced their IP for many years but were increasingly irritating practitioners and made them an example in the press of bad IP behavior. Around 2000, they [Athena] stopped enforcing and tried to develop their “Academic Partnership Program.” Although the intent was to allow laboratories to retain some volume for research and training of clinical laboratorians, it ultimately failed largely because if a laboratory did more than 100 cases in a year, the licensing fees made the laboratories noncompetitive” (M. Watson, personal communication, 2008).
We have clearly identified three instances of patent enforcement by Athena Diagnostics for Connexin 26 testing against other providers. The first of these proved to be a case of noninfringing use that has been resolved (R. Flaherty, BioReference Laboratories, personal communication, 2008; S. Bale, GeneDx, personal communication, 2008). In testimony before the House Judiciary Subcommittee on Courts, the Internet and Intellectual Property, on October 30, 2007, Marc Grodman, CEO of BioReference Laboratories (BRLI), indicated that while GeneDx (a company acquired by BRLI) was performing a genetic test for a rare skin condition by full sequence analysis of the gene in question, it “received a letter from another laboratory claiming that within the sequence being analyzed was another sequence associated with hearing loss.”48 Athena Diagnostics' letter indicated that since testing for this hearing loss gene was patented, performing the test might be an act of infringement. Attempts by GeneDx to perform the test by paying a royalty to the other company were unsuccessful. We have confirmed by personal communication with Dr. Grodman and Dr. Sherri Bale, Clinical Director at GeneDx, that the genetic test in question involved sequencing the Connexin 26 gene for mutations associated with a rare skin condition KID. Dr. Bale confirmed that Athena Diagnostics sent a notification letter and indicated that the matter has been resolved. “We accepted a letter from Athena that instructed us to not report the 35delG mutation. However, what we have done is if we find the deletion, we call the referring MD, tell them the results and that we can not report them, and then suggest they redraw the patient and send the sample to Athena for testing” (S. Bale, personal communication, 2008). This requires a second visit to the patient's physician, another blood draw, and payment, this time to Athena Diagnostics, to repeat the GJB2 test. (Appendix 6, Letter from Sherri Bale, GeneDx to Athena Diagnostics.)
GeneDx currently continues to perform full sequence analysis for Connexin 26 to identify the GJB2 D50N mutation and other mutations associated with a rare skin condition KID, which is not covered by the patents licensed to Athena. We understand the matter reached amicable resolution with GeneDx agreeing not to report hearing loss mutations and referring to Athena if they are found (Appendix 7). Athena Diagnostics, which holds the exclusive license to GJB2 mutation testing in the United States, expressed willingness to grant sublicenses (M. Henry, personal communication, 2007). However, according to Dr. Sherri Bale, Athena refused to grant a sublicense when GeneDx attempted to acquire one in the context of KID testing (R. Flaherty, personal communication; S. Bale, personal communication, 2008). This case also raises concerns about withholding of useful clinical information and increased costs, as another blood draw and test by Athena would be required if GeneDx identified a potential hearing loss mutation in a sample sent to them for KID testing, although this is clinically unlikely.
In another instance, the Diagnostic Molecular Pathology Laboratory at the University of California Los Angeles stopped offering testing for Connexin 26/GJB2 over 2 years ago, after receiving a letter from Athena Diagnostics. According to Dr. Wayne Grody, Director of the Laboratory, the terms of the sublicense offered by Athena Diagnostics “were unreasonable, with an upfront fee of $50,000 per year plus a significant per test fee” and not economically viable for the laboratory, given the relatively low volume of testing for hearing loss at UCLA (W. Grody, University of California Los Angeles, personal communication, 2008). Attempts to negotiate terms of a sublicense were not successful. It is unclear to what extent cessation of testing at UCLA has affected patient access to hearing loss testing. Dr. Grody indicated that samples are now sent to Athena Diagnostics for clinical testing. His laboratory considered using an alternate test methodology, namely custom ASRs from Third Wave Technologies for Connexin 26 mutation testing. This method reportedly allows laboratories to avoid infringing the Connexin 26 patents licensed to Athena. It is unclear if this is because a sublicense acquired from Athena Diagnostics comes attached to the purchase of the ASRs or because the test methodology (InvaderTM assay) offers “workarounds” of the patents (US5998147, 6485908). However, these ASRs are no longer being offered since HoloLogics Inc acquired Third Wave Technologies in June 2008.5
Dr. Grody indicated that even if the alternate methodology could help overcome the problem of patent infringement, it is not ideal because ASRs for the 235delC Connexin 26 mutation, found commonly in Asian populations, are not available from Third Wave. Testing for this mutation is particularly relevant at UCLA given the high Asian and Asian American population in California. Dr. Grody also noted that shipping samples to Athena Diagnostics is problematic for indigent patient populations covered by the California MediCaid program (MediCal). MediCal only reimburses laboratories with which it has a contract, which Athena does not have.
We also recently became aware that Athena Diagnostics sent a notification letter to the Center for Human Genetics at Boston University regarding testing for a number of genetic conditions including hearing loss (A. Milunsky, Boston University, personal communication, 2008). In August 2008, the Center for Human Genetics ceased testing for hearing loss and several other conditions for which Athena has exclusive IP rights. Athena confirmed that no sublicenses have been given to university and academic or medical centers (M. Henry, personal communication, 2007).
The SoundGeneTM panel offered by Pediatrix is performed under a sublicense from Athena Diagnostics for GJB2/Connexin 26 testing. To our knowledge, Pediatrix is the only provider that has received a sublicense from Athena Diagnostics to date. Presumably, this will lead to a steady royalty stream for Athena from genetic testing done by Pediatrix as part of newborn hearing loss screening, and a flow of patients for diagnostic follow up.
Microarray chip-based diagnostics for hearing loss are currently not available as a clinical service in the United States. However, if chip-based diagnostics do become commercialized, and if use of DNA probes on those microarrays infringe the patents that Athena has licensed, Athena Diagnostics could choose to demand a license for testing that includes patented sequences of Connexin 26 and MTRNR1. Simultaneous multi-gene testing also seems to be a departure from the current ACMG clinical guidelines, which call for a systematic utilization of genetic tests based on relative frequencies, family histories, patient symptoms, and apparent diagnosis. Those guidelines might change, however, if microarray testing proved equally sensitive, specific, and accurate, while being faster and cheaper and identifying many mutations in different genes in a single test.
Consumer utilization
This case study finds limited effects on patient access to genetic testing for hearing loss that can be directly attributed to patenting. The availability of genetic testing for hearing loss in California may be limited for MediCal patients because the patent-holder, Athena Diagnostics, lacks a contract with MediCal and is out-of-state. The issue here is not patents per se, but patents preventing other laboratories from offering the test under MediCal contract. The laboratories with MediCal contracts do not have sublicenses from Athena and Athena apparently does not have a contract with MediCal.
We were unable to identify systematic evidence beyond the MediCal situation noted above, that the patents have impeded utilization of hearing loss tests by people who are interested in or require testing. Testing for the genes licensed exclusively to Athena Diagnostics is not marketed directly to consumers by Athena or by other direct-to-consumer providers like DNAdirect. Sixteen providers other than Athena Diagnostics are listed on GeneTests.org as offering Connexin 26 testing. Nine providers in addition to Athena Diagnostics are listed for MTRNR1 testing. Many of these provider websites have detailed information on the availability and cost of both patented and unpatented hearing loss genetic tests. Although several providers for these tests have emerged, we found no information about usage of the tests by consumers.
Although we did not query test providers about their testing volume or the number of patients requesting each test, a future survey could assess utilization of hearing loss tests by consumers. It would also be valuable to determine how frequently reimbursement for such tests is denied by insurers and payers, as coverage and reimbursement of genetic testing are likely to affect consumer use.
Finally, patient access may be affected, as much or more by factors other than patents, such as the lack of knowledge about the genetics of hearing loss, particularly among primary care physicians, and their low propensity to refer cases for genetic testing as follow-up.49 A recent survey by Duncan et al.50 noted that while 86% of pediatric otolaryngologists reported having easy access to genetic testing services for referral, many also identified “discomfort with various aspects of genetic testing” as a reason for not ordering genetic tests. Lack of knowledge about genetic testing or about interpretation of test results may be a more significant barrier to test adoption by health care providers than patents.
Coverage and reimbursement by third party payers
We have no evidence that gene patents have directly affected third party payer coverage and reimbursement decisions for hearing loss tests. Laboratories report that insurers have generally adopted genetic testing for some hearing loss genes, as illustrated below by the coverage position from CIGNA HealthCare on “Genetic Testing for Congenital Profound Deafness.”
“CIGNA covers genetic testing for congenital, nonsyndromic, sensorineural, mild-to-profound deafness (DFNB1) as medically necessary for ANY of the following indications:
-
For diagnostic testing when the clinical examination and conventional studies suggest a diagnosis of congenital, nonsyndromic, sensorineural, mild-to-profound deafness (DFNB1).
-
1
For carrier testing in EITHER of the following situations:
-
a
When the individual has a first- or second-degree relative* with gene GJB2 or GJB6 mutation
-
b
When the individual is the reproductive partner of a known carrier (deafness-causing mutation of gene GJB2 or GJB6) and the couple has the capacity and intention to reproduce
-
c
For prenatal testing when both parents are known carriers of deafness-causing mutation of gene GJB2 or GJB6 mutation.
CIGNA does not cover genetic screening for congenital, nonsyndromic, sensorineural, mild-to-profound deafness (DFNB1) in the general population because such screening is considered not medically necessary or of unproven benefit.
All individuals undergoing genetic testing for any reason should have both pre- and post-test genetic counseling with a physician or licensed or certified genetic counselor.”51
Aetna covers full sequence and targeted mutation analysis of GJB2/Connexin 26 and deletion analysis for GJB6/Connexin 30, but it excludes preimplantation genetic diagnosis for DFNB1, which is deemed an “unproven benefit at this time.” We have not verified whether other commercial insurers have a similar position, except through the interviews with testing laboratories. Indirect effects that patents may have on price might lead to a higher level of scrutiny by insurance providers if the tests are priced above other genetic tests, but hearing loss genetic test prices are in the same range as other case studies. Decisions about coverage for SLC26A4, MTRNR1, and MTTS1 may be case by case, because these conditions are not common enough to warrant an explicit coverage policy. These tests are likely handled similarly to tests for other rare conditions, covering tests in a routine price range and requiring special justification for expensive testing. During the informal phone survey, most test providers indicated that hearing loss genetic tests were mostly covered by insurance. However, we have no direct evidence about how often consumers are denied coverage for hearing loss testing, pay for them out of pocket, face high copay fees because of reimbursement limits, or encounter other factors that affect their choice to get such tests.
Athena Diagnostics has a policy of directly billing insurance providers for services when Athena is the contracted provider for that particular plan. However, when Athena is not a contracted provider and the insurer does not cover the testing in part or full, Athena guarantees as part of its Patient Protection Plan that “an eligible, enrolled patient's liability will be limited to 20% of the cost of the test, even if the patient's insurance plan pays nothing. (These programs are discussed at greater length in the spinocerebellar ataxia case study.) For patients enrolled in the Patient Protection Plan, any amount collected from the insurance company in excess of 80% of the amount billed will be refunded to the patient.”52 The Patient Protection Plan is not, however, available in all states, does not apply to government health programs (Medicare and Medicaid, for example) and does not apply to most insurers and health plans. Florida and Maryland are excluded, for example.
Athena Diagnostics does not participate in Medicaid but it does offer discounts to Medicaid patients through its financial assistance programs. If the test of interest is not covered by Medicare carriers, the patient will be required to pay for the test in advance. In such cases, if the Medicare carrier denies coverage of the test, the patient may have to pay the entire cost out of pocket, because Medicare patients are ineligible for Athena's Patient Protection Plan. Thus, insurance coverage, independent of the patenting status of the test, may limit patient access in some cases, specifically Medicaid patients, most Medicare patients, and those covered by health plans with which Athena does not have a contract. However, even in these cases, patients have the option of using other providers who may accept Medicaid, at least as long as those providers continue to offer the service.
CONCLUSION
Patents do not appear to have significantly impeded patient or clinical access for hearing loss genetic testing. Many institutions provide tests, even those covered by patents exclusively licensed to Athena Diagnostics, presumably without a sublicense. Although Athena Diagnostics has sent out some notification letters, enforcement is apparently incomplete, as several other testing services are listed on GeneTests.org. It is possible that the volume of testing at most institutions, even for Connexin 26, is not large enough to warrant more aggressive enforcement by Athena Diagnostics.
Given that experts have recommended incorporation of genetic tests into EHDI programs, use of genetic tests for hearing loss is likely to increase. The recent introduction of the SoundGeneTM diagnostic panel by Pediatrix Screening is indicative of this trend. However, concerns have been raised that a small panel such as SoundGeneTM may not be ideal. For example, patients with GJB2-related hearing loss may be missed because they do not carry the mutations represented on the panel. More recent literature suggests that it is not sufficient to test only the four common mutations associated with Pendred syndrome included in the panel. This is one reason, many laboratories now sequence the entire SLC26A4 gene because targeted mutation testing misses many mutations. Multi-gene, chip-based tests may help address problems in diagnosing individuals who develop hearing loss as children or adolescents, and potentially reduce the cost and duration of diagnostic testing. These new diagnostics, although likely to detect a much broader range of mutations and gene variants, may also miss rare and novel mutations, especially for genes like GJB2 and SLC26A4, because patients often have new or private mutations. The clinical utility and analytical validity of such array-based tests also needs to be demonstrated. It remains to be seen whether patents on genes and mutations for hearing loss will impede development of multi-allele methods.
This case study illustrates the complexity of assessing the impact of patents on access to genetic testing. This is because of the number of genes and mutations involved and also depends on how patents are enforced. Aggressive patent enforcement might reduce the number of outlets for genetic testing, and for those not covered by health plans covering payment to Athena Diagnostics, this would reduce access. It therefore appears that patient access depends on an unstable intellectual property regime and the vicissitudes of payment contracts between health insurers and health care plans, on one hand, and different testing laboratories, on the other.
Genetic testing for hearing loss also illustrates several other features of intellectual property and genetic testing. Most of the patents for commonly tested genes are owned by academic institutions and licensed to Athena Diagnostics. The patenting and licensing practices of academic institutions are therefore linked to both the benefits and problems associated with having a single major provider. The case also illustrates the penumbra effect of exclusive rights to some mutations leveraging testing for others, although it is also clear from this case that the effect is incomplete because multiple providers continue to offer tests.
REFERENCES
Burton S, Withrow K, Arnos K, Kalfoglou A, Pandya A . A focus group study of consumer attitudes toward genetic testing and newborn screening for deafness. Genet Med 2006; 8: 779–783.
Taneja P, Pandya A, Foley D, Nicely L, Arnos K . Attitudes of deaf individuals towards genetic testing. Am J Med Genet 2004; 130: 17–21.
American College of Medical Genetics Genetics evaluation guidelines for the etiologic diagnosis of congenital hearing loss. Genetic Evaluation of Congenital Hearing Loss Expert Panel. ACMG statement. Genet Med 2002; 4: 162–171.
Heller M, Eisenberg R . Can patents deter innovation? The anticommons in biomedical research. Science 1998; 280: 698–701.
Transcript of a conference call held by Hologic, Inc on June 9, 2008; SEC Accession No. 0001193125-08-130599. San Francisco, CA: SEC, 2008. Available at: http://www.sec.gov/Archives/edgar/data/859737/0001193125-0-130599-index.htm. Accessed March 18, 2010.
Boston University and The Center for Hum Genet, Inc. Announce Change in Testing Services. BioMedicine News. Available at: http://www.bio-medicine.org/medicine-technology-1/Boston-University-and-The-Center-for-Human-Genetics–Inc–Announce-Change-in-Testing-Services-2851–1/. Accessed November 14, 2008.
Morton C, Nance W . Newborn hearing screening—a silent revolution. N Engl J Med 2006; 354: 2151–2164.
Kelsell D, Dunlop J, Stevens H, et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997; 387: 80–83.
Denoyelle F, Weil D, Maw M, et al. Prelingual deafness: high prevalence of 30delG mutation in the connexin 26 gene. Hum Mol Genet 1997; 6: 2173–2177.
Nanogen licenses rights to gene linked to hereditary deafness. Fresh News. 2003. Available at: http://www.freshnews.com/news/biotech-biomedical/article_15724.html?Nanogen. Accessed March 10, 2007.
Institut Pasteur Technology Transfer. Available at: http://www.pasteur.fr/ip/easysite/go/03b-000024–0ap/licensing-opportunities/list-oftechnologies-available-for-licencing-or-transfer. Accessed January 16, 2009.
Kenneson A, Myers M, Lubin I, Boyle C . Genetic laboratory practices related to testing of the GJB2 (connexin 26) gene in the United States in 1999 and 2000. Genet Test 2003; 7: 49–56.
Pandya A, Arnos K, Xia X, et al. Frequency and distribution of GJB2 (connexin 26) and GJB6 (connexin 30) mutations in a large North American repository of deaf probands. Genet Med 2003; 5: 295–303.
University of Cardiff. Human gene mutation database. Available at: http://www.hgmd.cf.ac.uk/ac/gene.php?gene=GJB2. Accessed November 19, 2008.
del Castillo I, Villamar M, Moreno-Pelayo M, et al. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N Engl J Med 2002; 346: 243–249.
Putcha G, Bejjani B, Bleoo S, et al. A multicenter study for the frequency and distribution of GJB2 and GJB6 mutations in a large North American cohort. Genet Med 2007; 9: 413–426.
Everett L, Glaser B, Beck J, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 1997; 17: 411–422.
Smith R, van Camp G . Pendred Syndrome/DFNB4. GeneReviews. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=pendred. Accessed April 15, 2007.
Cook-Deegan RM, DeRienzo C, Carbone J, Chandrasekharan S, Heaney C, Conover C . Impact of gene patents on access to genetic testing for inherited susceptibility to cancer: comparing breast and ovarian cancers to colon cancers. Genet Med. In press.
Pandya A Nonsyndromic hearing loss and deafness, mitochondrial. GeneReviews. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=mt-deafness. Accessed January 15, 2008.
Prezant T, Agapian J, Bohlman M, et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet 1993; 4: 289–294.
Estivill X, Govea N, Barcelo A, et al. Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment with aminoglycosides. Am J Hum Genet 1998; 62: 27–35.
Reid F, Vernham G, Jacobs H . A novel mitochondrial point mutation in a maternal pedigree with sensorineural deafness. Hum Mutat 1994; 3: 243–247.
Early identification of hearing impairment in infants and young children: National Institutes of Health Consensus Development Conference Statement. National Institutes of Health; March 1–3 1993. Available at: http://consensus.nih.gov/1993/1993HearingInfantsChildren092html.htm. Accessed January 16, 2009.
White K . The current status of EHDI programs in the United States. Ment Retard Dev Disabil Res Rev 2003; 9: 79–88.
National Resource Center for Early Hearing Detection and Intervention. Available at: http://www.infanthearing.org/tas/index.html. Accessed August, 2007.
Norris V, Arnos K, Hanks W, Xia X, Nance WE, Pandya A . Does universal newborn hearing screening identify all children with GJB2 (connexin 26) deafness? Penetrance of GJB2 deafness. Ear Hear 2006; 27: 732–741.
Arnos K . The implications of genetic testing for deafness. Ear Hear 2003; 24: 324–331.
White K . Early hearing detection and intervention programs: opportunities for genetic services. Am J Med Genet 2004; 130A: 29–36.
Schimmenti L, Martinez A, Fox M . Genetic testing as part of the early hearing detection and intervention (EHDI) process. Genet Med 2004; 6: 521–525.
Robin N, Prucka S, Woolley A, Smith R . The use of genetic testing in the evaluation of hearing impairment in a child. Curr Opin Pediatr 2005; 17: 709–712.
Connor C, Craig H, Raudenbush S, Heavner K, Zwolan T . The age at which young deaf children receive cochlear implants and their vocabulary and speech-production growth: is there an added value for early implantation?. Ear Hear 2006; 27: 628–644.
A white paper addressing the societal costs of hearing loss and issues in third party reimbursement. November 8, 2004. Available at: http://www.audiologyonline.com/articles/pf_article_detail.asp?article_id=1204. Accessed January 16, 2009.
Mohr P, Feldman J, Dunbar J, et al. The societal costs of severe to profound hearing loss in the United States. Int J Technol Assess Health Care 2000; 16: 1120–1135.
Preciado D, Lim L, Cohen A, et al. A diagnostic paradigm for childhood idiopathic sensorineural hearing loss. Otolaryngol Head Neck Surg 2004; 131: 804–809.
Fischel-Ghodsian N . Mitochondrial mutations and hearing loss: paradigm for mitochondrial genetics. Am J Hum Genet 1998; 62: 15–19.
Joint Committee on Infant Hearing (JCIH) Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 2000; 106: 798–817.
Pediatrix Medical Group. SoundGene Clinician Information. Available at: http://www.pediatrix.com/body.cfm?id=2889. Accessed January 16, 2009.
Siemering K, Manji S, Hutchison W, Du Sart D, Phelan D, Dahl H . Detection of mutations in genes associated with hearing loss using a microarray-based approach. J Mol Diagn 2006; 8: 483–489.
Gardner P, Oitmaa E, Messner A, Hoefsloot L, Metspalu A, Schrijver I . Simultaneous multigene mutation detection in patients with sensorineural hearing loss through a novel diagnostic microarray: a new approach for newborn screening follow-up. Pediatrics 2006; 118: 985–994.
Shumaker J, Metspalu A, Caskey C . Mutation detection by solid phase primer extension. Hum Mutat 1996; 7: 346–354.
Kurg A, Tonisson N, Georgiou I, Shumaker J, Tollett J, Metspalu A . Arrayed primer extension: solid-phase four-color DNA resequencing and mutation detection technology. Genet Test 2000; 4: 1–7.
Asper Biotech Hereditary hearing loss testing. Available at: http://www.asperbio.com/HHL.htm. Accessed March 10, 2007.
Asper Biotech Hereditary hearing loss tests supplement. Available at: www.asperbio.com/supplement.pdf. Accessed March 10, 2007.
Preciado D, Lawson L, Madden C, et al. Improved diagnostic effectiveness with a sequential diagnostic paradigm in idiopathic pediatric sensorineural hearing loss. Otol Neurotol 2005; 26: 610–615.
Computational Medical Center. Gene chip diagnostic test for pediatric hearing impairment. Available at: http://www.computationalmedicine.org/project/hearing_loss.htm. Accessed March 10, 2007.
Li CX, Pan Q, Guo YG, et al. Construction of a multiplex allele-specific PCR-based universal array (ASPUA) and its application to hearing loss screening. Hum Mutat 2008; 29: 306–314.
Statement of Marc Grodman at Stifling or Stimulating—The Role of Gene Patents in Research and Genetic Testing. House Judiciary Subcommittee on Courts, the Internet and Intellectual Property. Washington, DC; 2007. Available at: http://judiciary.house.gov/hearings/pdf/Grodman071030.pdf. Accessed February 4, 2010.
Moeller M, White K, Shisler L . Primary care physicians' knowledge, attitudes, and practices related to newborn hearing screening. Pediatrics 2006; 118: 1357–1370.
Duncan R, Prucka S, Wiatrak B, Smith R, Robin N . Pediatric otolaryngologists' use of genetic testing. Arch Otolaryngol Head Neck Surg 2007; 133: 231–236.
CIGNA HealthCare Cigna medical coverage policy. Available at: http://www.cigna.com/customer_care/healthcare_professional/coverage_positions/medical/mm_0254_coveragepositioncriteria_genetic_test_congenital_profound_deafness.pdf. Accessed January 16, 2009.
Athena Diagnostics Ordering and billing. Available at: http://www.athenadiagnostics.com/content/ordering/. Accessed August, 2007.
del Castillo I Moreno-Pelayo MA del Castillo FJ et al. Prevalence and evolutionary origins of the del(GJB6–D13S1830) mutation in the DFNB1 locus in hearing-impaired subjects: a multicenter study. Am J Hum Genet 2003; 73: 1452–1458
Acknowledgements
This case study was carried out under grant P50 003391, co-funded by the National Human Genome Research Institute and US Department of Energy, and supplemented by funding from The Duke Endowment. The case study authors have no consultancies, stock ownership, grants, or equity interests that would create financial conflicts of interest. The Center for Genome Ethics, Law & Policy accepts no industry funding. Dr. Robert Cook-Deegan is listed on the British Medical Journal roster of physicians who have pledged to remain independent of industry funding (http://www.tseed.com/pdfs/bmj.pdf); more details about how the case studies were done are noted in a 29 July 2009 letter to the Secretary's Advisory Committee on Genetics, Health, and Society (http://www.genome.duke.edu/centers/gelp/documents/SACGHSResponsetopubliccomments.pdf).
All interviews were conducted under Duke University Institutional Review Board-approved protocol 1277 and usually conducted by phone and recorded. Researchers obtained informed consent from subjects. These interviews are covered by an NIH certificate of confidentiality.
This case study was reviewed by Richard L. Faherty, Michael Henry, Wayne Grody, Iris Schrijver, Sherri Bale, Michael Hopkins, Ignacio del Castillo, and Michael Watson for the Secretary's Advisory Committee on Genetics, Health, and Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The authors declare no conflict of interest. See Acknowledgments for details.
Appendices
APPENDIX 1
APPENDIX 2
APPENDIX 3: UNIVERSAL NEWBORN HEARING SCREENING EDHI GUIDELINES FOR PEDIATRIC MEDICAL HOME PROVIDERS
See guidelines Available at: http://www.infanthearing.org/medicalhome/aap_gpmhp.pdf. Accessed January 16, 2009.
APPENDIX 4

APPENDIX 4: CINCINNATI CHILDREN′S HOSPITAL HEARING LOSS GENETIC EVALUATION CLINICAL GUIDELINES. Image copyright Cincinnati Children's Hospital Medical Center, used with permission.
APPENDIX 5
APPENDIX 6

APPENDIX 6: LETTER FROM GENEDX TO ATHENA DIAGNOSTICS REGARDING CONNEXIN 26 SEQUENCING
APPENDIX 7

APPENDIX 7: EMAIL RESPONSE FROM ATHENA DIAGNOSTICS TO GENE DX SHARED WITH PERMISSION OF DR. SHERRI BALE, CLINICAL DIRECTOR, GENEDX
Rights and permissions
About this article
Cite this article
Chandrasekharan, S., Fiffer, M. Impact of gene patents and licensing practices on access to genetic testing for hearing loss. Genet Med 12 (Suppl 1), S171–S193 (2010). https://doi.org/10.1097/GIM.0b013e3181d7b053
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e3181d7b053
Keywords
This article is cited by
-
Framing Ethical Concerns and Attitudes towards Human Gene Patents in the Chinese Press
Asian Bioethics Review (2020)
-
DNA patents and diagnostics: not a pretty picture
Nature Biotechnology (2010)