Abstract
Purpose
There is little known about the long-term efficacy and safety of Ahmed glaucoma valve (AGV) implant and about the conditions affecting surgical success in uveitic glaucoma (UG).
Patients and methods
The charts of adult patients with UG who underwent AGV implantation from 2006 to 2015 were reviewed retrospectively.
Results
Data of 46 eyes of 39 patients were evaluated. Mean follow-up was 51.93±23.08 months. Mean preoperative IOP was 37.05±9.62 mm Hg and mean number of preoperative topical anti-glaucomatous medications was 2.98±0.27. One eye (2%) was defined as failure because of implant extraction surgery. In the rest of the eyes, intraocular pressure (IOP) was under control with or without anti-glaucomatous medications during follow-up. The cumulative probability of complete success (IOP control without medications) was 78% at 6 months, 76% at 1 year, 71% at 2 years, 66% at 3 years, and 63% at 4 years (95% confidence interval, 61.24–87.81). The cumulative probability of eyes without complication was 64% at 6 months, 48% at 12 months, 44% at 24 months, 41% at 36 months, and 38% at 48 months (95% confidence interval, 34.64–62.85). Complete success was lower in eyes with previous ocular surgery than the eyes without (P=0.061) and it was lower in eyes with active inflammation at the time of surgery than the eyes without (P=0.011).
Conclusion
AGV implantation is an effective and safe alternative method in the management of UG, especially when it is performed as a primary surgical option and when no inflammation is present preoperatively.
Similar content being viewed by others
Introduction
Glaucoma is the third most common complication of uveitis after cystoid macular edema and cataract, and it is seen in 10–20% of uveitic patients.1, 2 The underlying mechanism of uveitic glaucoma (UG) has not been fully understood.1 Etiology of uveitis, inflammatory mechanisms, and steroid treatment have impacts on glaucoma development.1 Medical treatment of UG is frequently insufficient and surgery is needed in a significant number of patients (23.2%).3 UG has special considerations compared with other types of glaucomas because inflammation has important effects both on surgical success and on postoperative complications.1
Trabeculectomy is the most widely performed surgical procedure in refractory glaucomas, but its long-term success rate is limited in UG because of early bleb failure secondary to accelerated healing response.2 Landers et al4 reported 20-year results of trabeculectomy in different types of glaucomas and they have found that UG has significantly lower success rate after trabeculectomy than other types of glaucomas. Uveitic eyes are also prone to develop hypotony in the early postoperative period secondary to ciliary body shutdown.1 So, both trabeculectomy and non-valved glaucoma drainage devices (GDDs), such as Molteno and Baerveldt implants, have a risk of early hypotony and related complications (such as choroidal detachment, flat anterior chamber (AC), and hypotony maculopathy).5, 6 Valved GDDs, such as Ahmed glaucoma valve (AGV), appear to be more successful in preventing early hypotony and in improving surgical success in patients with UG.7, 8
Several studies have addressed the results of AGV implantation in UG.8, 9, 10, 11, 12, 13, 14 Most of them were small case series and reported short to intermediate-term outcomes (mean postoperative follow-up was 14.2–31.7 months). Their reported success rates are also variable (from 50 to 94%). Previously we have reported short-term to long-term results of AGV implantation in the management of UG secondary to Behçet disease.15 The aim of the current study was to present the efficacy and safety of AGV implantation in the management of refractory glaucoma secondary to other different uveitis etiologies in adults and to assess the conditions affecting surgical success.
Subjects and methods
The study was conducted by the tertiary Glaucoma and Uveitis-Behçet Clinics of Ankara Training and Research Hospital. The charts of adult patients who underwent AGV implantation for UG from 2006 to 2015 and reached at least 6 months of postoperative follow-up were reviewed retrospectively. Patients’ demographic data and medical history were recorded. Complete ophthalmologic examination, including best-corrected distance visual acuity with Snellen charts, intraocular pressure (IOP) measurement with Goldmann applanation tonometer, and dilated fundus examination were performed at each visit. AC cellular activity was scored according to the SUN Working Group criteria.16 Data from the preoperative examination was used as the baseline examination for entry in the study. At least 3 months of period without inflammation was awaited before surgery. But in some cases, this time period could not be waited because of impending glaucomatous optic neuropathy.
The protocol of the study was designed according to World Glaucoma Association guidelines on design and reporting of glaucoma surgical trials,17 approved by the Review Board of the hospital, and adhered to the tenets of the Helsinki declaration.
Surgical technique
A fornix-based conjunctiva and Tenon capsule flap was created at the superotemporal quadrant of the eye and an AGV (model FP7 or S2, New World Medical Inc., Rancho Cucamonga, CA, USA) was used in all eyes. Before implantation, the tube was primed with balanced salt solution using a 26-gauge blunt cannula. The anterior edge of the plate was sutured to the sclera 8–10 mm posterior from the limbus. At 2 mm posterior to the limbus, a 22-gauge needle was used to enter the AC. The tube was trimmed for correct size and inserted into the AC through the needle track. No pars plana insertion of the tube was performed in any of the eyes. The tube was fixed to the sclera using 2 interrupted 10/0 nylon sutures and covered with bovine pericardium (Tutoplast, Tutogen Medical, Alachua, FL, USA). The pericardium was fixed to the sclera with interrupted 10/0 nylon sutures. Ultimately the conjunctiva was closed with 10/0 nylon sutures. No antifibrotic agent (such as mitomycin-C or fluorouracil) was used in any of the eyes during the surgery.
Postoperative treatment
At the end of the surgery, subconjunctival injection of dexamethasone was performed in each eye. At the postoperative period, each patient received topical prednisolone acetate (1%) eye drops hourly, ketorolac tromethamin (0.5%, or nepafenac (0.1%) eye drops four times daily while awake, and oral fluocortolone 1 mg/kg for 3 days, and followed by a tapering dose according to the inflammatory status of the eye. Each eye received topical ofloxacin (0.3%) or moxifloksacin (0.5%) eye drops eight times daily, especially during the first week after surgery. The patients who had viral etiology received oral acyclovir 400 mg two times daily started 1 week before surgery and continued postoperatively according to the patients’ clinical status. Systemic immunosuppressive agents were continued if the patient had been receiving these before surgery.
Success and failure criteria
Success was defined as having IOP between 6 and 21 mm Hg with (qualified success) or without (complete success) anti-glaucomatous medications and no need for further glaucoma surgery or tube extraction surgery. Overall success was defined as the sum of complete and qualified success. Tube revision surgeries were not defined as failure if the tube was still in place and IOP was under control postoperatively. Hypotony was defined as having IOP ≤5 mm Hg. Complications within the first month after AGV implantation were defined as early complications. Early hypotony was not defined as surgical failure.
Statistical analysis
Data was analyzed using SPSS 17 statistical software (SPSS for Windows, Chicago, IL, USA). In descriptive statistics, mean values±SD (range) were used for quantitative variables as frequency distributions for categorical data. χ2-test was used to test differences between categorized data. Wilcoxon signed-rank test was used to test means between two dependent and Mann–Whitney U-test was used for two independent variables. The cumulative survival rates were calculated using Kaplan–Meier life-table analysis. Survival curves of subgroups were compared with the log-rank test. P-values of <0.05 were considered to be statistically significant.
Results
During the study period, 50 eyes of 43 patients were underwent AGV implantation for UG. Four eyes of four patients were not included in the study because they did not reach 6 months of follow-up. The study included 46 eyes of 39 patients (22 females and 17 males). The sample included 8 eyes (17.4%) with idiopathic anterior uveitis, 8 eyes (17.4%) with uveitis secondary to ankylosing spondilitis, 8 eyes (17.4%) with Fuchs heterochromic iridocyclitis, 7 eyes (15.2%) with pars planitis, 5 eyes (10.9%) with viral anterior uveitis, and 10 eyes (21.7%) with other etiologies (Table 1).
Mean age at the time of AGV implantation was 39.17±12.15 years (range, 19–66 years). Mean postoperative follow-up after surgery was 51.93±23.08 months (range, 6–108 months). Mean preoperative IOP was 37.05±9.62 mm Hg (range, 23–60 mm Hg) and mean number of preoperative topical anti-glaucomatous medications was 2.98±0.27 (range, 2–3). Preoperatively in 88.4% of the patients were using oral acetazolamide. Mean duration of preoperative topical beta blocker use was 22.13±30.89 months, carbonic anhydrase inhibitor (CAI) use was 19.36±28.09 months, and alpha adrenergic agonist use was 13.71±20.73 months. Topical prostaglandin analogs were not used in any of the eyes because of their potential of inducing uveitis exacerbation and cystoid macular edema in patients with uveitis.18
Mean time without inflammation before surgery was 7.07±9.00 months (range, 0–45 months). In 6 eyes (13.04%), there was active inflammation (2+ aqueous cells) at the time of surgery. In 8 eyes (17.39%), there was uveitic relapse within 3 months prior to surgery, but no active inflammation at the time of surgery, and in the rest of 32 eyes (69.57%), 3 months of quiescence of inflammation was present before the surgery.
Eleven eyes (23.9%) had undergone at least one ocular surgery prior to AGV implantation (Table 1). Before AGV implantation, cataract was present in 13 eyes (28.3%), 8 eyes (17.4%) were pseudophakic, and the rest of 25 eyes (54.3%) had normal lenses (Table 1).
Complications
Ocular complications and their managements are summarized in Table 2. The most prevalent early complication was hypotony, which was encountered in 12 eyes (26.1%) and spontaneous recovery was observed in all eyes.
One month after surgery, the most frequent complication was cataract progression in 25 of 38 eyes (65.8%) that were phakic preoperatively. Twenty three of these eyes (92.0%) underwent cataract extraction after a mean time of 16.25±15.57 months (range, 2–48 months) from AGV implantation. Mean time from AGV implantation to cataract extraction was 9.54±11.09 months (range, 2–38 months) in eyes that had cataract preoperatively and it was 24.30±16.85 months (range, 3–48 months) in eyes that had normal lenses preoperatively (P=0.012). For cataract extraction, a standard phacoemulsification surgery was performed and no additional therapeutic measures were taken except the postoperative treatment regime for uveitic eyes as described in patients and methods section.
Partial occlusion of the tube with iris was observed in 2 eyes (4.3%). One of these eyes underwent revision surgery because of uncontrollable IOP levels with ocular hypotensive agents. No revision surgery was performed in the other eye because IOP was under control with medications thorough follow-up.
Tube exposure was observed in 1 eye (2.2%) 5 months after AGV implantation and revision surgery was performed. One month after revision, a second tube exposure with endophthalmitis was developed. The implant was removed and the patient was treated with intravitreal antibiotics (vancomycin and ceftazidime).
In the whole sample, the cumulative probability of eyes without complication was 64.4% at 6 months, 48.3% at 12 months, 43.7% at 24 months, 41.3% at 36 months, and 38.1% at 48 months (95% confidence interval, 34.64–62.85) after surgery based on Kaplan–Meier survival analysis (Figure 1a).
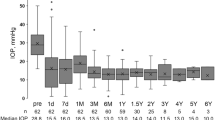
Percent survival patients without a complication after Ahmed glaucoma valve implantation (a). Box-plot representation of IOP values over 6 years of follow-up (b): Median values (dark lines), 25/75 (boxes), and 5/95 percentiles (bars), and outliers (circles), respectively. Follow-up periods and number of cases in each period are shown on the abscissa. Upper dashed line represents 21 mm Hg and lower dashed line represents 6 mm Hg.
Success
According to our success criteria, 1 eye (2.2%) was defined as failure because implant extraction surgery was performed. Changes in IOP, number of anti-glaucomatous medications, and the percentage of eyes under anti-glaucomatous medications at each time point are summarized in Table 3 and IOP changes are demonstrated in Figure 1b.
Although mean number of anti-glaucomatous medications remained similar during follow-up, mean IOP was decreased from 15.36±3.67 mm Hg (range, 8–21 mm Hg) at 6 months to 11.86±1.35 mm Hg (range, 10–14 mm Hg) at 6 years (P=0.042).
The cumulative probability of complete success among the whole sample was 78.3% at 6 months, 76.0% at 1 year, 71.4% at 2 years, 66.5% at 3 years, and 63.6% at 4 years (95% confidence interval, 61.24–87.81) based on Kaplan–Meier survival analysis (Figure 2a). When stratified for uveitis etiology, the least complete success rate was observed in the eyes with uveitis secondary to ankylosing spondilitis, while the highest success rate was observed in the eyes with pars planitis, but the difference did not reach statistical significance (P=0.241, Figure 2b).
Survival curve for complete success among the whole sample (a), stratified for uveitis etiology (b), previous ocular surgery (c), and preoperative inflammatory status (d). AS, ankylosing spondilitis; AU, idiopathic anterior uveitis; FHIC, Fuchs heterochromic iridocyclitis; PP, pars planitis; VAU, viral anterior uveitis.
The cumulative probability of complete success was higher in eyes without previous ocular surgery than the eyes with previous ocular surgery (P=0.061, Figure 2c). The eyes with active inflammation at the time of AGV implantation had significantly lower complete success rate than the eyes without active inflammation at the time of surgery (P=0.011, Figure 2d).
No significant difference was found in the duration of preoperative topical beta blocker, alpha adrenergic agonist, and CAI use between complete and qualified success groups (P=0.22, 0.36 and 0.86, respectively).
Discussion
It is difficult to make comparisons between glaucoma surgical trials because of variability in study populations, in study designs, and in the definition of surgical success. AGV implant has been shown as an effective and safe method in the management of UG, but the reported success rates are highly variable.8, 9, 10, 11, 12, 13, 14 In our study, IOP was under control in all eyes with or without anti-glaucomatous medications except 1 eye having tube extraction surgery. Percentage of eyes without anti-glaucomatous medications in IOP control in previous studies differs from 26 to 67% after AGV implantation in UG.9, 10, 11, 13, 14 After AGV implantation, mean IOP was decreased significantly during follow-up although mean number of anti-glaucomatous medications remained similar (Table 3, Figure 1b). This was in contrast with the study of Ozdal et al11 in which they did not find a significant difference in IOP during follow-up after AGV implantation in UG.
Success rate of AGV implantation between different uveitis etiologies has never been compared before. In this study, although it did not reach statistical significance, eyes with pars planitis had the highest and eyes with ankylosing spondilitis had the lowest complete success rates (Figure 2b).
Having previous ocular surgery is a well-known risk factor for trabeculectomy failure, but its effect on AGV success is not clear.4 Gil-Carrasco et al9 found no effect of previous ocular surgery on AGV success in UG, but only 3 eyes (21.4%) without previous ocular surgery were enrolled in their study. Satana et al13 found no significant difference in surgical success between eyes with secondary AGV implantation after previously failed trabeculectomy and eyes with primary AGV implantation in patients with Behçet disease. Bettis et al8 reported that eyes with prior cataract surgery have a non-significant trend toward decreased overall success of AGV implantation in UG. Like this, in our study, eyes with previous ocular surgery had a tendency to have lower complete success rate than the eyes without previous ocular surgery, although the difference could not reach statistical significance (P=0.061, Figure 2c).
Inflammation has been described as one of the most important reasons for trabeculectomy failure in patients with uveitis.4, 19 The effect of inflammation on AGV success is not clear. Some authors suggest that strict control of inflammation before AGV implantation is mandatory.8, 10, 11 But Gil-Carrasco et al9 found no effect of preoperative degree of inflammation on overall AGV success in UG. In our study, AGV implantation was performed while active inflammation was present in 6 eyes (13.0%). Eyes with inflammation at the time of AGV implantation significantly needed anti-glaucomatous medications more frequently in controlling IOP (lower complete success rate) than eyes without inflammation (P=0.011, Figure 2d). The mechanism of action of inflammation on AGV success is not clear. The inflammatory cells and debris at the time of surgery may have an adverse effect on valve mechanism or on proper encapsulation of the plate.
Topical anti-glaucomatous medications lead to subclinical conjunctival inflammation.20 Increased number and duration of preoperative topical anti-glaucomatous medications are associated with trabeculectomy failure.4, 20, 21 The effect of preoperative topical anti-glaucomatous use on AGV success was also investigated in this study. The number of preoperative topical anti-glaucomatous medication was almost same in all eyes, and no difference was found between complete and qualified success groups according to the duration of preoperative beta blocker, alpha adrenergic agonist, and CAI use.
Hypotony is the most frequently reported complication in the early postoperative period after AGV implantation in UG (from 4.2 to 42.8%).8, 9, 10, 11, 12, 13 Hyphema is the other reported complication in the early postoperative period, which was reported in up to 21.4% of the eyes and it is usually self-limited. Like previous studies the most frequent early complication was hypotony in 12 eyes (26.1%) in our study. In 9 eyes, hypotony was mild and presented with only shallow AC, while hypotony maculopathy developed in 2 eyes (4.3%) and hyphema was accompanied in 1 eye (2.2%). No surgical intervention was needed and spontaneous recovery was observed in all eyes. No hypotony was observed 1 month after surgery. No choroidal detachment secondary to hypotony was observed in our study compared with other authors’ reports of which reported choroidal detachment up to 7.1% of the eyes after AGV implantation in UG.9, 11, 12
Cataract progression was the most frequent complication and cataract surgery was the most frequently performed ocular surgery 1 month after AGV implantation in this study (Table 2). The exact contribution of the AGV implant on the progression of cataract is hard to calculate because both inflammation and steroid treatment have effects on cataract progression. It is not possible to compare the cataract rate of our study with previous studies because most of the previous studies included complicated cases in which cataract surgery was performed before or at the time of AGV implantation in the majority of eyes.8, 9, 10, 11, 14
Tube occlusion secondary to hemorrhage, fibrovascular ingrowth, with iris or vitreous have been reported in up to 26.3% of eyes.8, 9, 10, 11, 12, 13 In our series, tube occlusion with iris was observed in 2 eyes (4.3%). One eye underwent revision surgery because of uncontrollable IOP with maximal topical anti-glaucomatous medications, while in the other eye the IOP remained under control with medications.
Exposure of the implant (tube or plate) is a potentially serious complication that can lead to endophthalmitis. Implant exposure was reported in up to 19.9% of the eyes after AGV implantation in UG.8, 9, 11, 12 Rachmiel et al12 reported that tube removal because of exposed implant was significantly more common in patients with UG than in patients with open-angle glaucoma. In our study, tube exposure was observed in 1 eye (2.2%). Conjunctival repair was performed with the use of amniotic membrane as described previously.22 One month after repair, the patient was presented with a second tube exposure and endophthalmitis. The implant was removed and the patient was successfully treated with intravitreal antibiotics.
Encapsulated cystic bleb was reported in up to 42.8% of eyes.9, 13 In our study, it was observed in 1 eye (2.2%). Capsulectomy of the Tenon capsule was performed because of uncontrollable IOP with medications. Corneal complications such as corneal-tube touch, corneal edema, corneal decompensation, and Dellen formation were reported in up to 7.14% of eyes.9, 10, 11, 12, 13 Corneal edema was observed in 1 eye (2.2%) and other corneal complications were not observed in any of the eyes in our series.
Other reported complications, such as wound dehiscence, retinal detachment, diplopia, ocular motility disturbances, dysesthesia, and pitosis were not observed in any of the eyes. Complications were most frequently encountered within the first 12 months and no complications were observed after 48 months of follow-up (Figure 1a).
In conclusion, this study is limited by its retrospective and non-comparative design, nevertheless it is one of the largest case series with AGV implantation in UG with the longest follow-up reported. The high success and low complication rates of our study may come from that we performed AGV implantation mostly in virgin eyes in which 76.1% of the eyes had no previous ocular surgery (Table 1). Previous studies mostly investigated AGV implantation in complicated cases and the rate of the eyes with previous ocular surgery differs from 68% to 81%.8, 9, 10, 11, 12, 13, 14 Best surgical option in the management of UG is still debatable. This study shows that AGV implantation is an effective and safe alternative method in the management of UG, especially when it is performed as a primary surgical option and in eyes without inflammation at the time of surgery.

References
Baneke AJ, Lim KS, Stanford M . The pathogenesis of raised intraocular pressure in uveitis. Curr Eye Res 2016; 41: 137–149.
Din NM, Isa H, Taylor SR, Barton K, Lightman SL . Intraocular pressure elevation in uveitis. Expert Rev Ophthalmol 2012; 7: 45–59.
Neri P, Azuara-Blanco A, Forrester JV . Incidence of glaucoma in patients with uveitis. J Glaucoma 2004; 13: 461–465.
Landers J, Martin K, Sarkies N, Bourne R, Watson P . A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology 2012; 119: 694–702.
Hill RA, Nguyen QH, Baerveldt G, Forster DJ, Minckler DS, Rao N et al. Trabeculectomy and Molteno implantation for glaucomas associated with uveitis. Ophthalmology 1993; 100: 903–908.
Ceballos EM, Parrish RK 2nd, Schiffman JC . Outcome of Baerveldt glaucoma drainage implants for the treatment of uveitic glaucoma. Ophthalmology 2002; 109: 2256–2260.
Sung VC, Barton K . Management of inflammatory glaucomas. Curr Opin Ophthalmol 2004; 15: 136–140.
Bettis DI, Morshedi RG, Chaya C, Goldsmith J, Crandall A, Zabriskie N . Trabeculectomy With mitomycin C or Ahmed valve implantation in eyes with uveitic glaucoma. J Glaucoma 2015; 24: 591–599.
Gil-Carrasco F, Salinas-VanOrman E, Recillas-Gispert C, Paczka JA, Gilbert ME, Arellanes-Garcia L . Ahmed valve implant for uncontrolled uveitic glaucoma. Ocul Immunol Inflamm 1998; 6: 27–37.
Da Mata A, Burk SE, Netland PA, Baltatzis S, Christen W, Foster CS . Management of uveitic glaucoma with Ahmed glaucoma valve implantation. Ophthalmology 1999; 106: 2168–2172.
Ozdal PC, Vianna RN, Deschênes J . Ahmed valve implantation in glaucoma secondary to chronic uveitis. Eye 2006; 20: 178–183.
Rachmiel R, Trope GE, Buys YM, Flanagan JG, Chipman ML . Ahmed glaucoma valve implantation in uveitic glaucoma versus open-angle glaucoma patients. Can J Ophthalmol 2008; 43: 462–467.
Satana B, Yalvac IS, Sungur G, Eksioglu U, Basarir B, Altan C et al. Ahmed glaucoma valve implantation for uveitic glaucoma secondary to Behçet disease. J Glaucoma 2015; 24: 607–612.
Papadaki TG, Zacharopoulos IP, Pasquale LR, Christen WB, Netland PA, Foster CS . Long-term results of Ahmed glaucoma valve implantation for uveitic glaucoma. Am J Ophthalmol 2007; 144: 62–69.
Yakin M, Eksioglu U, Sungur G, Satana B, Demirok G, Ornek F . Short-term to long-term results of Ahmed Glaucoma Valve implantation for uveitic glaucoma secondary to Behçet disease. J Glaucoma 2017; 26: 20–26.
Jabs DA, Nussenblatt RB, Rosenbaum JT ; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005; 140: 509–516.
Heuer DK, Barton K, Grehn F, Shaarawy T, Sherwood M . Consensus on definition of success. In: Shaarway T, Sherwood M, Grehn F (eds). Guidelines on Design and Reporting of Glaucoma Surgical Trials: World Glaucoma Association. Kugler Publications: Amsterdam, The Netherlands, 2009, p 17.
Horsley MB, Chen TC . The use of prostaglandin analogs in the uveitic patient. Semin Ophthalmol 2011; 26: 285–289.
Stavrou P, Murray PI . Long-term follow-up of trabeculectomy without antimetabolites in patients with uveitis. Am J Ophthalmol 1999; 128: 434–439.
Broadway DC, Grierson I, O’Brien C, Hitchings RA . Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol 1994; 112: 1446–1454.
Lavin MJ, Wormald RP, Migdal CS, Hitchings RA . The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol 1990; 108: 1543–1548.
Ainsworth G, Rotchford A, Dua HS, King AJ . A novel use of amniotic membrane in the management of tube exposure following glaucoma tube shunt surgery. Br J Ophthalmol 2006; 90: 417–419.
Acknowledgements
MY has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis as well as the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sungur, G., Yakin, M., Eksioglu, U. et al. Assessment of conditions affecting surgical success of Ahmed glaucoma valve implants in glaucoma secondary to different uveitis etiologies in adults. Eye 31, 1435–1442 (2017). https://doi.org/10.1038/eye.2017.84
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2017.84
This article is cited by
-
Prognostic factors for successful Baerveldt glaucoma implant surgery for refractory glaucoma after multiple surgeries
Japanese Journal of Ophthalmology (2021)
-
Efficacy of glaucoma drainage devices in uveitic glaucoma and a meta-analysis of the literature
Graefe's Archive for Clinical and Experimental Ophthalmology (2019)