Abstract
Purpose
Tissue clearing has been used in anatomy for the first time in Germany over a century ago. Neuronal tissue, like cortex, was investigated in mice using a water-based optical clearing method termed See Deep Brain (SeeDB). However, although the eye belongs to the central nervous system, this histological technique was not applied in the eye up to date. We applied SeeDB for the visualization of intraocular structures.
Patients and methods
Four eyes of cornea donors (two male, two female: 73–84 years) obtained from the Cornea Bank of the Department of Ophthalmology Erlangen, four chicken eyes and two mices’ optic nerve were used. Bulbi were fixed in 4% paraformaldehyde in phosphate-buffered saline and treated with increasing concentrations of aqueous fructose solution with 0.5% α-thioglycerol. After SeeDB, transscleral macrophotographs of the choroid were performed.
Results
Complete transparency of the sclera was obtained in enucleated human and chicken eyes after SeeDB treatment. Macroscopical anatomy of the choroid (partially transparent due to the remaining retinal pigment epithelium and melanocytes) showing vessels and other related structures was possible without preparing slides. Mice optic nerves were also transparent after SeeDB treatment.
Conclusion
The SeeDB method allows visualization of intraocular structures through a completely translucent sclera. This innovative processing technique could facilitate comprehensive qualitative and quantitative topographical anatomical studies of human and animal eyes, preserving their 3D architecture. Supra- and intrachoroidal ganglionic plexus could potentially be visualized transsclerally. Finally, clinical-pathological correlations of intraocular diseases—for example, retinal tumors—will be possible in non-dissected eyes.
Similar content being viewed by others
Introduction
Tissue clearing has been investigated for a long time. Already in 1914, Spalteholz1 reported for the first time about a method for clarifying tissues. Since then it was the aim of several investigators to look inside structures without opening them and therefore destroying their intrinsic anatomy by increasing transparency. For this purpose, it is necessary to minimize light scattering, which is caused by several internal cellular structures (eg, membranes and nuclei) and extracellular material (eg, extracellular matrix). One approach for clearing biological structures is to dehydrate and treat it with solvents, continuing Spalteholz’s technique.2, 3, 4 Methanol, which is the most common dehydrant agent2, 3 and other like benzyl benzoate, methylsalicylate, or dichlormethane2, 3, 4, 5, 6 solve lipid structures. However, the main disadvantages of these techniques are the shrinkage of the treated tissue due to the dehydration,5 the toxic properties of several solvents, and the limited use for subsequent fluorescent markers.7 Therefore, especially in the last years a second technique, based on water, became more and more popular.8, 9 This method removes lipids and reduces the refractive index by hyperhydration (Sca/e, Clear T, and CUBIC)8, 10, 11 or hydrogel embedding (CLARITY, PACT, and PARS).12, 13 A further option for clearing tissues is immersion based. Aqueous solutions with molecules of high refractive index (eg, fructose) lead to a clarifying of the tissue. Using this technique, lipids are not removed and fluorescent methods or protein labeling is still possible.7 In addition, especially small samples can be treated. Ke et al14 introduced for the first time the See Deep Brain method (SeeDB), using this immersion-based technique with fructose for clearing cerebral tissue. SeeDB works without increasing tissue volume and alteration of lipids or proteins, thus allowing subsequent fluorescent techniques.
Although the eye is related to cranial structures, SeeDB was not used for ocular tissues until now. Applying the SeeDB technique, intraocular structures could be potentially visualized transsclerally. This could open new possibilities for the macroscopic and histological visualization of the normal anatomy or pathological changes of the eye maintaining original architecture. The aim of this study was to use the immersion technique SeeDB for clearing ocular tissues of human and chicken as well as mices’ optic nerve.
Materials and methods
Four eyes of cornea donors (two male, two female: 73–84 years) were obtained from the Cornea Bank of the Department of Ophthalmology Erlangen-Nürnberg, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU). After trephination of the corneoscleral button with appropriate research consent, the eyes were fixed by 4% paraformaldehyde (PFA, pH 7.2–7.4, 2.5 h at room temperature, RT, Roth, Karlsruhe, Germany), nitrogen-cooled in methylbutane at −80 °C and stored at −20 °C. In addition, four chicken adult eyes were used. The chickens (Gallus gallus, type Ross) were decapitated in the context of an agricultural slaughtering at a local poultry farm. Eyes were fixed by 4% PFA and transported on ice to the laboratory. All eyes were nitrogen-cooled in methylbutane at −80 °C and stored at −20 °C. One mouse (C57BI/6JRi) was anaesthesized by isoflouran and killed by cervical dislocation. The eyes with the whole optic nerve were unfixed nitrogen-cooled methylbutane at −80 °C and stored at −20 °C.
Human and chicken eyes were opened along the ora serrata and cut into an anterior and posterior part, divided by the iris. A solution of 20–100% fructose, filled up to 20 ml with distilled water, was mixed in a shaker at 65 °C. After cooling to 25–37 °C, 0.5% α-thioglycerol was added. Mice and chicken globes incubated in the diverse fructose solution at room temperature:
-
20% fructose (4 g), 100 μl thioglycerol for 7 h
-
40% fructose (8 g), 100 μl thioglycerol for 16 h
-
60% fructose (12 g), 100 μl thioglycerol for 8 h
-
80% fructose (16 g), 100 μl thioglycerol for 16 h
-
100% fructose (20 g), 100 μl thioglycerol for 25 h.
Finally, specimens were incubated in SeeDB solution, containing 20.25 g fructose, solved in 5 ml distilled water, and 100 μl thioglycerol, for 23 h at room temperature.
Human globes were incubated in the diverse fructose solution at 37 °C:
-
20% fructose (4 g), 100 μl thioglycerol for 4 h
-
40% fructose (8 g), 100 μl thioglycerol for 4 h
-
60% fructose (12 g), 100 μl thioglycerol for 4 h
-
80% fructose (16 g), 100 μl thioglycerol for 12 h
-
100% fructose (20 g), 100 μl thioglycerol for 12 h.
Finally, specimens were incubated in SeeDB solution, containing 20.25 g fructose, solved in 5 ml distilled water, and 100 μl thioglycerol, for 24 h at 37 °C.
After optical clearing, transscleral macrophotographs were taken by macroscopic digital photography at room temperature.
Results
Clearing of chicken oculi
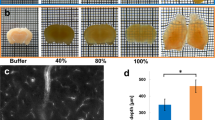
Half of chicken globes were photographed before (Figure 1a and c) and after immersion with SeeDB (Figures 1b and d), which worked in chicken with diverse effects on different ocular tissues:
(1) SeeDB did not alter the normal configuration of ocular structures. (2) Scleral tissue cleared totally. (3) Choroidal vessels and the pecten oculi remained in their normal configuration and could be visualized transclerally. (4) Choroidal transparency was only partial, because the SeeDB did not cause depigmentation of uveal melanocytes (Figures 4e and f). (5) SeeDB did not influence the color of retinal pigment epithelium. (6) Finally, iris went partially clear (Figures 4c and d).
Clearing of human oculi
After immersion with SeeDB human eyes were analyzed compared to the tissue before the treatment:
(1) Incubation with SeeDB did not alter markedly the form and volume of the tissue. Only a fine alteration in volume (shrinkage <5%) was observed: 22.29 × 22.80 × 0.56 mm (before treatment) and 21.34 × 21.76 × 0.54 mm (after incubation with SeeDB). (2) The sclera got transparent (Figures 2a–d). (3) Choroidea could be seen throughout the sclera from outside (Figures 3a–d) and was partially clear due to the remaining pigmentation of uveal melanocytes (Figures 4g and h), as observed in chicken eyes. (4) Also retinal pigment epithelium remained pigmented.
Human eyes: quarter of the eye before (a) and after treatment with SeeDB (b–d); exterior view (a, b; N, nervus optici): sclera went totally transparent; choroid is visible throughout the sclera; interior view (c) with folded choroid and exterior view (d): folded choroid can be seen throughout the sclera.
Clearing of mouse’s optic nerve
Application of SeeDB on mouse optic nerve showed a notable increase of axons’ transparency (Figures 4a and b, arrow).
Discussion
Clearing human or animal tissues in their own entirety is the aim of many studies since its first trial in 1914. In the following years, it was the intention of several studies to improve the method by avoiding shrinking or enabling the application for subsequent methods (eg, immunolabeling). Organs or biological structures show their intrinsic function best in their normal surrounding. SeeDB, an immersion-based technique, uses high refractive index solutions for clarifying tissues with only less morphological distortion.14, 15 Until now, there is no report in literature about the potential use of SeeDB in the eye. It was the purpose of this study to applicate the optical clearing method SeeDB on diverse ocular human and animal tissues.
Transparency can be achieved by reducing light scattering. Light, passing through the tissue, is deflected many times. In living organisms, cells are surrounded by extracellular fluid and matrix. There light is scattered in many different directions due to presence of cell bodies, membranes, and intracellular organelles. Particularly, short-wavelength light is often absorbed and re-emitted by molecules, being smaller than the wavelength of visible light, known this phenomenon as ‘Rayleigh scatter’. In contrast, the ‘Mie scatter’ occurs at larges anatomical structures (ie, organelles), scattering all light independently of its wavelength. It is the purpose of all clarifying methods to ‘bundle’ this scattering in one direction. Only the light, which passes a vacuum, has no scatter at all. Refractive index is the result of dividing the velocity of the light passing a vacuum by the velocity of light in a specific sample. But not only the different refractive indexes of diverse tissues generate the scatter—also the inhomogeneity of natural organisms, independently of their refractive indexes, produces it.7
Studies investigating clearing of ocular tissues can be found rarely in literature. Several approaches were done using local or systemic glycerol for clarifying rabbit sclera.16 Dehydration and crosslinking, an irreversible method, was applied also on sclera.17 The sclera consists of a dense network of extracellular matrix, containing microfibrils with a diameter of 30–300 nm bathed by extracellular fluid.18 It is proposed that high refractive fluids (eg, fructose in SeeDB) replace the interstitial fluid with consecutive interruption of hydrogen bonding. This exchange results in a reversible optical clearing of the sclera as observed for cerebral tissues.19
Next to clarifying sclera, neuronal samples can be clarified using SeeDB. Appling it, mice optic nerves became transparent. This finding is consistent with the data obtained from cerebral tissue, investigated by Ke et al.14 Applying SeeDB on mouse brain increased transparency allowing a visualization depth of >1000 μm using confocal microscopy.20 This aqueous-based method seems to work well at small organs like the eye. However, larger or thicker samples would need an alternative clearing method, which may use higher concentrations of detergents. One great benefit of the SeeDB method is the potential reversibility of the application. In addition, this method works quickly and is easy to handle. Next to its less costs, it requires no special laboratory equipment.14
The disadvantage of many clearing techniques, which removed proteins and therefore avoid subsequent immunolabeling, can be prevented applying the clearing CLARITY method (Clear, Lipid-exchanged, Acrylamide-hybridized Rigid, Imaging/immunostaining compatible, Tissue hydrogel). Tissues embedded in hydrogel and detergents or electrophoresis, removes lipids. Glycerol immersion finalizes the protocol.12, 21 Also developing brain can be visualized as transparent structure by an improved version of CLARITY.22
Up to now, for example, rat, mouse, and human tissue were successfully clarified.20, 21 However, no investigations were made on chicken samples, neither with SeeDB, nor with any other clearing technique. In our hands, SeeDB worked well on chicken eyes. Sclera went totally clear. Choroids and iris got partially transparent due to the remaining choroidal and iridal melanocytes. Bleaching of retinal pigment epithelium was not observed. Clearing especially chicken eyes is of interest in ophthalmic research, because it served as animal model for myopia development.
Research into techniques for clarifying animal and human structures will certainly increase in the next years. The focus is to enable subsequent methods, such as immunostaining or tracing techniques on cleared tissues, to work successfully. These developments are of special interest to visualize the ‘normal’ topography of diverse cells, thereby getting information about their potential function. Typically, the thicker the tissue, the greater the challenge is to develop a successful clearing method. In the last years, adaptive optics were introduced in the imaging of thick samples, however its application was limited.23 Yet a potential combination of clearing methods (eg, SeeDB) with adaptive optics will yield a greater success. Especially in the eye the exact location and form of intraocular structures in un-opened eyes is of interest for pathologists, considering benign or malign tumors. Clearing methods are not only of interest for several clinical approaches, but also for research studies. Investigation on specific tracking of nerve processes in its own entirety or reconstruction of tissue in their ‘original’ three-dimensional architecture without disruption of it needs previous clearing techniques to work well. Single-photon confocal microscopy on embryonic specimen could be facilitated after clearing of the samples. In addition, myopia research will be promoted by investigating choroidal innervation, topography, and growth of the axial length in their ‘natural’ surrounding.
Conclusion
Clearing human and chicken sclera as well as mice optic nerves are possible using SeeDB. This opens the possibility of investigation of intraocular structures as choroid and retina without alteration of their 3D anatomical architecture. This quick and easy to handle technique would enable application of subsequent histological methods, for example, immunolabeling.

References
Spalteholz W . Über das Durchsichtigmachen von tierischen und menschlichen Präparaten und seine theoretische Bedingungen, nebst Anhang: Über Knochenfärbung. Hirzel; Leipzig, 1914.
Dodt HU, Leischner U, Schierloh A, Jährling N, Mauch CP, Deininger K et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat Methods 2007; 4: 331–336.
Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M . iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 2014; 159: 896–910.
Ertürk A, Becker K, Jährling N, Mauch CP, Hojer CD, Egen JG et al. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc 2012; 7: 1983–1995.
Becker K, Jährling N, Saghafi S, Weiler R, Dodt HU . Chemical clearing and dehydration of GFP expressing mouse brains. PLoS One 2012; 7: e33916.
Steinke H, Wolff W . A modified Spalteholz technique with preservation of the histology. Ann Anat 2001; 183: 91–95.
Richardson DS, Lichtmann JW . Clarifying tissue clearing. Cell 2015; 162: 246–257.
Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C . ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development 2013; 140: 1364–1368.
Hou B, Zhang D, Zhao S, Wei M, Yang Z, Wang S et al. Scalable and DiI-compatible optical clearance of the mammalian brain. Front Neuroanat 2015; 9: 19.
Hama H, Kurokawa H, Kawano H, Ando R, Shimogori T, Noda H et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci 2011; 14: 1481–1488.
Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 2014; 157: 726–739.
Chung K, Deisseroth K . CLARITY for mapping the nervous system. Nat Methods 2013; 10: 508–513.
Yang B, Treweek JB, Kulkarni RP, Deverman BE, Chen CK, Lubeck E et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 2014; 158: 945–958.
Ke MT, Fujimoto S, Imai T . SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci 2013; 16: 1154–1161.
Neu CP, Novak T, Gilliland KF, Marshall P, Calve S . Optical clearing in collagen- and proteoglycan-rich osteochondral tissues. Osteoarthritis Cartilage 2015; 23: 405–413.
Zaman RT, Rajaram N, Nichols BS, Rylander 3rd HG, Wang T, Tunnell JW et al. Changes in morphology and optical properties of sclera and choroidal layers due to hyperosmotic agent. J Biomed Opt 2011; 16: 077008.
Tanaka Y, Kubota A, Yamato M, Okano T, Nishida K . Irreversible optical clearing of sclera by dehydration and cross-linking. Biomaterials 2011; 32: 1080–1090.
Meek KM, Fullwood NJ . Corneal and scleral collagens–a microscopist’s perspective. Micron 2001; 32: 261–272.
Yeh AT, Hirshburg J . Molecular interactions of exogenous chemical agents with collagen–implications for tissue optical clearing. J Biomed Opt 2006; 11: 014003.
Ke MT, Imai T . Optical clearing of fixed brain samples using SeeDB. Curr Protoc Neurosci 2014; 66: Unit 2.22.
Tomer R, Ye L, Hsueh B, Deisseroth K . Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat Protoc 2014; 9: 1682–1697.
Zheng H, Rinaman L . Simplified. CLARITY for visualizing immunofluorescence labeling in the developing rat brain. Brain Struct Funct 2016; 221: 2375–2383.
Booth MJ . Adaptive optics in microscopy. Philos Trans A Math Phys Eng Sci 2007; 365: 2829–2843.
Acknowledgements
We thank Professor Dr W Neuhuber for his laboratory support. The present work was performed in partial fulfillment of the requirements for obtaining the degree, Dr rer. biol. hum. ‘(Hohberger B)’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hohberger, B., Baumgart, C. & Bergua, A. Optical clearing of the eye using the See Deep Brain technique. Eye 31, 1496–1502 (2017). https://doi.org/10.1038/eye.2017.83
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2017.83
This article is cited by
-
Light sheet fluorescence microscopy of cleared human eyes
Communications Biology (2023)