Abstract
Purpose
Ranibizumab is used in the treatment of choroidal neovascularization (CNV). Although systemic exposure to ranibizumab is low after ocular administration, its mechanism of action must be regarded as potentially teratogenic and embryo-fetotoxic. Women are advised to wait 3 months after the last dose of treatment with ranibizumab before conceiving. Little is known about the fetal side-effects of this drug.
Methods
Three pregnant women were treated with ranibizumab. One patient had idiopathic CNV.
Results
After receiving injections at 10 and 21 weeks after her last menstrual period (LMP), she gave birth to a healthy child. The second patient had myopic choroidal neovascularization (mCNV) and was treated by a single injection at 17 weeks post LMP. She gave birth to a healthy child after an uneventful pregnancy. The third patient had CNV secondary to a punctuate inner choroiditis. The injection was performed at 8 weeks post LMP. This patient presented a cholestasis of pregnancy at 36 weeks post LMP and gave birth at 38 weeks post LMP to a child that did not present any malformations.
Conclusions
This case series describes three women who underwent intravitreal ranibizumab treatment during pregnancy without showing any obstetric, embryofetal or neonatal complications.
Similar content being viewed by others
Introduction
Anti-vascular endothelial growth factor (VEGF) molecules are commonly used in the treatment of choroidal neovascularization (CNV) and macular edema. VEGF plays a pivotal role during pregnancy, and systemic anti-VEGF administration during this period should thus be avoided. However, the fetal risk of intravitreal injection during pregnancy has not been evaluated to date. Ranibizumab is an anti-VEGF that has been used for over a decade. Here, we present three cases of patients treated with intravitreal ranibizumab during their pregnancy.
Case reports
Patient 1
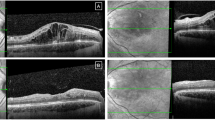
A 26-year-old woman with a medical background of miscarriage suffered an exudative recurrence of idiopathic CNV, manifested by blurred vision and metamorphopsia. The best corrected visual acuity (BCVA) corresponded to a Snellen score of 5/10. Optical coherence tomography (OCT) revealed macular edema with serous macular neurosensory detachment (SMND). With the consent of the patient, an intravitreal ranibizumab injection was administered at 10 weeks post LMP. A second injection was administered at 21 weeks post LMP.
Patient 2
A 31-year-old woman, pregnant via in vitro fertilization and suffering from myopic CNV of the right eye, presented an active recurrence with a clinical BVCA Snellen score of 5/10 and macular edema with SMND, revealed by OCT. She received an injection at 17 weeks post LMP.
Patient 3
A 30-year-old woman with a complex obstetric history suffered a recurrence of a punctuate inner choroiditis of the right eye. BCVA obtained a Snellen value of 6/10. Macular OCT confirmed the recurrence of exudation. An intravitreal injection of ranibizumab was performed at 8 weeks post LMP. Cholestasis of pregnancy occurred at 35 weeks after LMP+6 days leading to an induced labor 2 weeks later.
The three patients gave birth to healthy children at full term by post-vaginal delivery. No malformations or neonatal diseases were observed for the children, who were of normal fetal size and weight.
Discussion
Ranibizumab is a recombinant humanized monoclonal antibody fragment targeted against human VEGF-A.
VEGF is expressed in multiple embryonic and fetal tissues during development, with the highest levels found in the lung, kidney, and heart. The increasing expression of this protein in placental tissues and fetal membranes throughout gestation suggests that it may play a role in embryofetal and placental development and in the maintenance of placental vascular function during pregnancy. VEGF may also be involved in the regulation of amniotic fluid volume and composition.1
In animal studies, a low incidence of skeletal abnormalities and shortened supernumerary ribs was observed in monkey fetuses after the pregnant mothers were treated with 1 mg/eye of ranibizumab. The 1 mg/eye dose resulted in serum levels of ranibizumab up to 13 times higher than the Cmax levels predicted for single eye treatment in humans. After the intravitreal ranibizumab treatment of the mothers at a dose providing an exposure equivalent to single eye treatment in humans (0.125 mg/eye), no skeletal abnormalities were observed in the fetuses.2
It is not known if ranibizumab and bevacizumab cross the human placenta. Although ranibizumab is a smaller molecule than bevacizumab (48 kDa vs 149 kDa), its transplacental passage rate is likely much lower than that of bevacizumab due to the inability of the Fab fragment to cross the placenta.
Intravenous bevacizumab has been shown to be embryotoxic and teratogenic when administered to rabbits, which present decreases in maternal and fetal body weights, an increased number of fetal resorptions and an increased incidence of fetal skeletal malformations.3
To the best of our knowledge, there are no published cases of pregnant woman treated with ranibizumab injection during the first two trimesters of pregnancy. Two cases of ranibizumab treatment during the third trimester of pregnancy4, 5 concerned two 29-year-old women who gave birth to healthy children after ranibizumab treatment for choroidal neovascularization. Nineteen cases of injections of bevacizumab during pregnancy have been published,6 with complications occurring during the first month of pregnancy in five cases.
Conclusion
To the best of our knowledge, this is the first report on the absence of embryofetal abnormalities following intravitreal injections of ranibizumab performed in three pregnant women in the early stages of pregnancy. Unfortunately, this finding does not qualify the treatment as safe. It would be necessary to treat thousands of women to exclude any risk. The benefit/risk needs to be highlighted with the patient, in particular during the first two terms of pregnancy. The treatment should be used with great caution and should only be applied if visual function and/or anatomical exudation have worsened during follow-up. Written informed consent from the patient is mandatory.

References
Cheung C. Y . Vascular endothelial growth factor: possible role in fetal development and placental function. J Soc Gynecol Investig 1997; 4: 169–177.
Highlights of prescribing information Lucentis; 8.1 use in specific populations: pregnancy, April 2017, approved by FDA. Available athttps://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125156s105lbl.pdf (accessed on 3rd January 2018).
Briggs Gerald G., Freeman Roger K., Yaffe Sumner J . Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, 2015.
Jouve Léa, Jad Akesbi, Jean-Philippe Nordmann . Safety and efficacy of ranibizumab for pregnant women in idiopathic choroidal neovascularization. Acta Ophthalmol 2015; 93: e597–e598.
Sarhianaki A., Katsimpris A., Petropoulos I. K., Livieratou A., Theoulakis P. E., Katsimpris. J. M . Intravitreal administration of ranibizumab for idiopathic choroidal neovascularization in a pregnant woman. Klin Monbl Augenheilkd 2012; 229: 451–453.
Polizzi Silvio, Mahajan V. B . Intravitreal anti-VEGF injections in pregnancy: case series and review of literature. J Ocul Pharmacol Ther 2015; 31: 605–610.
Acknowledgements
We thank Dr Miriam Balstad for reading this paper and giving us her valuable advises.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Michel Weber receives fees as a consultant for the company Novartis. The other authors do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Fossum, P., Couret, C., Briend, B. et al. Safety of intravitreal injection of ranibizumab in early pregnancy: a series of three cases. Eye 32, 830–832 (2018). https://doi.org/10.1038/eye.2017.305
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2017.305
This article is cited by
-
Intravitreal anti-vascular endothelial growth factor injections in pregnancy and breastfeeding: a case series and systematic review of the literature
Eye (2023)
-
Suprachoroidal triamcinolone versus posterior subtenon triamcinolone either alone or formulated in the management of diabetic macular edema
International Ophthalmology (2023)
-
Intravitreal anti-vascular endothelial growth factor medications during pregnancy: current perspective
International Ophthalmology (2021)