Abstract
Purpose
To assess the short-term and long-term visual outcomes in patients with choroidal neovascularisation (CNV) secondary to angioid streaks treated with intravitreal anti-vascular endothelial growth factor (VEGF).
Methods
Retrospective, single-centre study.
Results
Overall 66 eyes of 52 patients were analysed. Follow-up ranged from 1 to 10 years. BCVA was 62 ETDRS letters at baseline, 68 letters at 1 year, 60 ETDRS letters at 5 years and 58 letters at 7 years. At 2 years patients gained 5.7 ETDRS letters from baseline but this gain was lost at 5 years. At 5 years there was an average loss of ETDRS letters from baseline of 3.3 letters. Sub-group analysis of subfoveal CNV showed worse outcome compared with eyes with extrafoveal and juxtafoveal CNV. In subfoveal CNV, BCVA was 53 ETDRS letters at 1 year (p < 0.0001) and 39 ETDRS at 5 years (p = 0.0005).
Conclusion
Anti-VEGF therapy is effective at stabilising visual acuity in patients with choroidal neovascularisation secondary to angiod streaks, however there is a gradual decline in visual acuity observed with 5–10 years of follow-up. Furthermore, subfoveal CNV have worse visual outcome compared with extrafoveal and juxtafoveal CNV.
Similar content being viewed by others
Introduction
Angioid streaks represent linear breaks within a calcified and brittle Bruch’s membrane. Clinically they appear as irregular bands with varying width that radiate from the optic nerve and are often bilateral. The presence of angioid streaks may be idiopathic or secondary to systemic disorders including pseudoxanthoma elasticum, Paget’s disease, Ehlers–Danos syndrome and hemoglobinopathies [1].
Choroidal neovascularisation (CNV) is a significant sight threatening complication of angioid streaks as the interrupted mechanical integrity of Bruch’s membrane creates a gateway for aberrant neovascularisation [2, 3]. CNV is estimated to occur in 72–86% of patients [4, 5] with bilateral involvement in up to 71% of patients [6]. The natural history of angioid streak associated CNV typically leads to profound visual loss and often legal blindness. Various treatment modalities have been utilised in the past with limited success including laser photocoagulation, photodynamic therapy with verteporin and surgical intervention. Currently intravitreal anti-vascular endothelial growth factor (VEGF) is the treatment of choice as these agents have been shown to improve or stabilise the visual acuity [7, 8]. However most of the evidence is from small case series or prospective studies with short-term follow-up and there is limited long-term data on visual outcomes and the efficacy of this treatment.
The primary aim of this study is to describe the short-term (1–2 years) and long-term (5–10 years) visual outcomes in patients treated with intravitreal anti-VEGF in a real world clinical setting. This study represents one of the largest retrospective reviews with the longest follow-up reported in literature to date, on the treatment of CNV secondary to angioid streaks with anti-VEGF therapy.
Subjects and methods
This study is a retrospective, non-comparative review of patients treated for CNV secondary to angioid streaks at Moorfields Eye Hospital, London, UK between January 2007 and December 2018.
The study adhered to the tenets of Declaration of Helsinki and was approved by the Clinical Effectiveness Committee at the hospital (CA18/MR/22–197).
Consecutive patients with CNV secondary to angioid streaks were identified from the electronic medical records at Moorfields eye hospital and only eyes which were treatment naive and had at least 12 months of follow-up were included. Patients who had concomitant ocular pathology that affected the best corrected visual acuity (BCVA) were excluded.
Data collected included patient demographics and BCVA at baseline and follow-up at 1, 2, 5, 7 and 10 years (converted to ETDRS letters for statistical analysis). The treatment regime including the type of anti-VEGF drug, number of injections and number of recurrences was also recorded. Recurrence of CNV was defined as recommencement of anti-VEGF therapy after 6 months or longer with no intervention. Ancillary tests were also reviewed by two independent reviewers to determine the angiographic classification and the location of the CNV in relation to the fovea and angioid streak.
Results
The study analysed 66 eyes of 52 patients (21 female and 31 male). The mean age at presentation was 52 years (range 18–82). The angioid streaks were secondary to pseudoxanthoma elasticum in 33 patients, sickle cell disease in one patient and abetaproteinemia in one patient. In the remaining 17 patients, there was no information regarding systemic association.
The location of the CNV was subfoveal in 21 eyes, juxtafoveal in 25 eyes and extrafoveal in 20 eyes. Fluorescein angiogram showed classic Type 2 lesions in 50 eyes (76%) and occult Type 1 lesion in 8 eyes. The CNV was identified adjacent to the angioid streak on FFA in 41 eyes (62%) (Table 1).
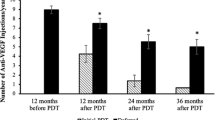
Bevacizumab was used as the initial agent in 64 eyes, 11 of which were then switched to ranibizumab or aflibercept. Ranibizumab was used as the initial agent in two eyes. Patients were treated in a pro re nata regime based on clinical evaluation and OCT findings. The mean number of injections was 5.5 in the 1st year, 2.7 in the 2nd year and 5.3 between the 3rd and 5th year. In patients who had 5 or more years of follow-up, a recurrence rate of 70% was observed with an average of 3 recurrences.
Mean BCVA of all patients at baseline was 62 ETDRS letters (6/19). At 1 and 2 year follow-up, the BCVA was 68 ETDRS letters (6/15) and 67 ETDRS letters (6/15), respectively. For patients who had 5 or greater years of follow-up, the mean BCVA was 60 ETDRS letters (6/19) at 5 years, 58 ETDRS letters (6/24) at 7 years and 66 ETDRS letters (6/15) at 10 years. On average patients gained 5.7 ETDRS letters in the first 2 years. After 5 years there was an average loss of ETDRS letters from baseline of 3.3 letters. The decline in visual acuity was also observed at 7 and 10 years, although this was not statistically significant (Table 2).
Sub-group analysis of the 21 eyes with subfoveal CNV showed worse outcome compared with eyes with extrafoveal and juxtafoveal CNV. Eyes with subfoveal CNV had a BCVA of 53 ETDRS letters (6/30) at 1 year follow-up while eyes with extrafoveal and juxtafoveal CNV had a BCVA of 75 ETDRS letters (6/10) (p < 0.0001). At 5 years follow-up the BCVA was 39 ETDRS (6/48) in eyes with subfoveal CNV while the BCVA was 68 ETDRS letters (6/15) in eyes with extrafoveal and juxtafoveal CNV (p = 0.0005). Patients with subfoveal CNV gained 4.6 letters in the first year of treatment but showed a gradual loss of ETDRS letters from baseline in the ensuing years (Table 3).
On presentation, 19 patients had poor visual acuity (BCVA ≤ 6/60) in the contralateral eye from a previous CNV. Two patients had a BCVA between 6/18 and 6/36 in the contralateral eye from a previous CNV. Seventeen patients developed CNV in the contralateral eye within the review period, however three of these patients were not included in the analysis as they did not have 12 months of follow-up. Overall, we observed bilateral disease in 38 of the 52 patients (73%).
Adverse events recorded over the 10-year period were minimal. One patient had an ischaemic cerebrovascular event 4 months after injection with bevacizumab for idiopathic angioid streaks at the age of 54. Another patient had an ischaemic coronary vascular event 2 weeks after intravitreal bevacizumab at the age of 63. There is limited information on the cardiovascular risk factors and family history of these two patients. A young female patient had three bevacizumab injections during pregnancy starting at 24 weeks gestation with no adverse effect on the foetus or pregnancy.
Discussion
CNV is a severe and prevalent complication of angioid streaks. It often impacts a relatively young working population and has a poor visual prognosis if left untreated. In the last decade treatment with anti-VEGF therapy has revolutionised the management of CNV of all aetiologies and short-term data has been promising in the subset of CNV secondary to angioid streaks.
In our series, with maximum follow-up of 10 years, treatment with anti-VEGF showed overall stabilisation of visual acuity (±10 ETDRS letters). However, the small gain in BCVA achieved in the 1st and 2nd year was lost by the 5th year. Between the 5th and 10th year we observed a small decline in ETDRS letters from baseline. Although there are no other studies describing visual outcomes with 5–10 years of data, evaluation of the limited literature does suggest a gradual decline in vision with time. Tilleul et al. showed in their case series that while treatment with ranibizumab was effective at maintaining BCVA, the visual acuity outcomes achieved at 2 years were not maintained at 4 years. Their results showed that 48.6% of eyes maintained visual acuity (±2 lines) with loss of VA (≥3 lines) occurring in 37.1% of eyes at final follow-up (mean of 48 months) [9]. The PIXEL study which evaluated treatment with Ranibizumab in PXE patients, showed at 2 years (n = 44) 77.3% of eyes maintained BCVA (±15 letters), with loss of VA (>15 letters) occurring in 11.4% of eyes. At 4 year follow-up, although the sample size was smaller consisting of 19 eyes, they showed a similar trend as BCVA was maintained in only 52.6% of eyes and 26.3% showed loss of VA (>15 letters) [10].
In the sub-group analysis of subfoveal CNV, the visual outcomes were less favourable compared with extrafoveal and juxtafoveal CNV. The mean BCVA at baseline was 48 ETDRS letters (6/38) and while there was a small gain in BCVA at 12 months, this was not maintained at 2 years. At 5 years follow-up eyes with subfoveal CNV had a BCVA of 39 ETDRS letters (6/48), which was significantly worse compared with a BCVA of 68 ETDRS letters (6/15) in eyes with extrafoveal and juxtafoveal CNV (p = 0.0005). This was in concordance with the findings of Giacomelli et al. In their case series, they showed that at 36 month follow-up, ten eyes with extrafoveal CNV lost 0.18 logmar, while 13 eyes with subfoveal CNV lost 0.59 logmar. They found the linear trend in visual acuity to be significant (p < 0.001) suggesting a worse outcome for eyes with subfoveal lesions [11].
The gradual decline in the visual acuity and the worse outcome for subfoveal CNV may be due to a number of factors including the inexorable nature of the disease. One significant factor is the high recurrence rate. We observed that the rate of recurrence was 70% in patients with 5 or more years of follow-up with an average of 3 recurrences. A period of 6 months of quiescence and no treatment was used to determine recurrence of disease in this review. This timeframe was proposed by the authors to ensure true recurrence of the disease and eliminate any false-positive results from patients who had prolonged disease activity or were under-treated in a PRN regime. We do however acknowledge that we may have omitted patients with recurrent disease at earlier periods of 4 or 5 months and hence the true recurrence rate may be greater than 70%.
The challenge with recurrence lies not only in the need for prompt treatment but also the aggressive nature of the CNV in this subset as demonstrated by Iacono et al. In their case series of non-foveal CNV, they observed recurrence with extension of the CNV toward the fovea in 1/3 of the patients by the 3rd year suggesting a high growth rate of the CNV [12]. Furthermore, they observed that while the BCVA was maintained at 1 year in non-foveal CNV, there was a decline in BCVA at 3 years. Their data illustrates that the rapid growth pattern of recurrences can inflict a more detrimental effect on the visual acuity.
Atrophy of the retinal pigment epithelium is also a paramount factor leading to decline in visual acuity. These atrophic changes can occur due to the natural history of the disease, but has also been hypothesised to be accelerated by anti-VEGF treatment as the breaks in Bruch’s membrane allow high permeability of the drug into the choriocapillaris [13, 14]. It is postulated that high concentrations of anti-VEGF drug in the densely vascular choriocapillaris can lead to atrophy with subsequent degeneration of the apically located RPE. Finger et al. showed in his series of PXE patients treated with bevacizumab that the size of the RPE atrophy and retinal fibrosis increased in both the treated and contralateral eye from baseline to follow-up [15]. However, given the small number of gradable images, there was no comparison whether the rate of atrophy was greater in the treated eye.
Treatment and monitoring protocol may also be a contributory factor to loss of BCVA over time. A PRN regime is the widely accepted treatment regime for most non-age related macular degeneration (AMD) CNV, however a PRN regime relies on patients’ early recognition of symptoms and immediate self-referral. Furthermore, the decision to treat is at the discretion of the consulting doctor and there is no consensus on long-term follow-up. In our study, fewer injections were administered in the 2nd year and 3rd to 5th year, compared with 5.5 injection administered in the first year. While the fewer injections over time correlated with worse visual acuity, this was not statistically significant. We also observed heterogeneity in the follow-up protocol but we did not analyse this to determine its effect on visual outcome. However, it can be inferred that in a disease where recurrence is a hallmark, a PRN regime with a lax follow-up protocol may struggle to achieve visual stabilisation.
Our study has strength in the relatively robust sample size and the long period of follow-up. The limitations of the study however lie in the retrospective nature lending to an inherent selection bias and the absence of a control group. Conducting a randomised controlled clinical trial however is not feasible as with-holding treatment for CNV in the current era of effective anti-VEGF therapy is not viable. Furthermore, given that angioid streaks are rare, large-scale prospective trials are also difficult to conduct.
CNV secondary to angioid streaks is a chronic disease and remains a therapeutic challenge. The perplexity in management arises from the younger demographic that is affected, coupled with the high rate of recurrence and the natural progression of fibrosis and atrophic changes. Treatment with anti-VEGF therapy however is safe and effective in stabilising and slowing the progression of the disease. Our findings emphasise the need for close follow-up in the long term to be able to detect early recurrence and treat promptly. Furthermore, close monitoring of the contralateral eye is also crucial in preserving vision.
Summary
Anti-VEGF therapy is effective at stabilising choroidal neovascularisations in angioid streaks, however long-term data shows a high rate of recurrence and gradual decline in vision over time. Furthermore, subfoveal CNV have worse visual outcome compared with juxtafoveal and extrafoveal CNV.
What was known before
-
Anti-VEGF therapy is effective for CNV for AMD.
-
Limited evidence shows efficacy of anti-VEGF for CNV secondary to angled streaks.
What this study adds
-
Shows what the long-term effects of anti-VEGF for CNV secondary to angioid streaks.
-
Despite treatment, there is a gradual decline in VA from baseline in CNV secondary to angioid streak treated with anti-VEGF.
References
Mansour AM, Ansari NH, Shields JA, Annesley WH Jr, Cronin CM, Stock EL. Evolution of angiod streaks. Ophthalmologica. 1993;207:57–61.
Yanoff M, Duker JS. Ophthalmology. 3rd ed. St. Louis, MO: Mosby Elsevier; 2009. p.707–9.
Gal-Or O, Balaratnasingam C, Freund KB. Optical coherence tomography angiography findings of choroidal neovascularization in pseudoxanthoma elasticum. Int J Retin Vitreous. 2015;1:11.
Al-Rashaed S, Arevalo JF. Long-term follow-up of choroidal neovascularization secondary to angioid streaks: case series and literature review. Clin Ophthalmol. 2012;6:1029–34.
Vaz-Pereira S, Collaco L, DeSalvo G, Van Zellar P. Intravitreal aflibercept for choroidal neovascularisation in angioid streaks. Eye. 2016;30:896.
Lim JI, Bressler NM, Marsh MJ, Bressler SB. Laser treatment of choroidal neovascularisation in patients with angioid streaks. Am J Ophthalmol. 1993;116:414–23.
Chatziralli I, Saitakis G, Dimitriou E, Chatzirallis A, Stoungioti S, Theodossiadis G, et al. Angioid streaks: a comprehensive review from pathophysiology to treatment. Retina. 2019;39:1–11.
Gliem M, Finger RP, Fimmers R, Brinkmann CK, Holz FG, Charbel IP. Treatment of choroidal neovascularisation due to angiod streaks: a comprehensive review. Retina. 2013;33:1300–14.
Tilleul J, Mimoun G, Querques G, Puche N, Zerbib J, Lalloum F, et al. Intravitreal ranibizumab for choroidal neovascularization in angioid streaks: four-year follow-up. Retina. 2016;36:483–91.
Mimoun G, Ebran JM, Grenet T, Donati A, Cohen SY, Ponthieux A. Ranibizumab for choroidal neovascularization secondary to pseudoxanthoma elasticum: 4-year results from the PIXEL study in France. Graefes Arch Clin Exp Ophthalmol. 2017;255:1651–60.
Giacomelli G, Finocchio L, Biagini I, Sodi A, Murro V, Introini U, et al. Long-term follow-up of choroidal neovascularization due to angioid streaks with pro re nata intravitreal anti-VEGF treatment. Ophthalmologica. 2017;238:44–51.
Iacono P, Parodi MB, La Spina C, Bandello F. Intravitreal bevacizumab for nonsubfoveal choroidal neovascularization associated with angioid streaks: 3-year follow-up study. Am J Ophthalmol. 2016;165:174–8.
Finger RP, Charbel IP, Ladewig M, Gotting C, Holz FG, Scholl HP. Fundus autofluorescence in pseudoxanthoma elasticum. Retina. 2009;29:1496–505.
Gibran SK, Sachdev A, Stappler T, Newsome R, Wong D, Hiscott P. Histological findings of a choroidal neovascular membrane removed at the time of macular translocation in a patient previous treated with intravitreal bevacizumab treatment (Avastin). Br J Ophthalmol. 2007;91:602–4.
Finger RP, Charbel IP, Schmitz-Valckenberg S, Holz FG, Scholl HN. Long-term effectiveness of intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum. Retina. 2011;31:1268–78.
Acknowledgements
I would like to thank the Moorfields Medical Retina Group for their contribution and collation of patients. The research was supported by the NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TR and SC have no proprietary interest. SS has received research grants, travel grants, speaker fees and attended advisory board meeting of Novartis, Bayer, Allergan, Roche, Boehringer Ingelheim, Optos Plc, Heidelberg Engineering.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramakrishnan, T., Chandra, S. & Sivaprasad, S. Long-term follow-up of management of choroidal neovascularisation secondary to angioid streaks with intravitreal anti-vascular endothelial growth factor. Eye 35, 853–857 (2021). https://doi.org/10.1038/s41433-020-0979-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0979-9