Abstract
Purpose
To determine the sensitivity and specificity of various methods of detecting a relative afferent pupillary defect (RAPD) in patients with glaucoma-related diagnoses.
Patients and methods
Patients underwent RAPD evaluation using the swinging flashlight method (SFM), the magnifier-assisted SFM, and pupillography using the Konan RAPDx. Main outcome measures were sensitivity and specificity of three methods of RAPD evaluation in detecting visual field mean deviation (MD), cup to disc ratio (CDR), disc damage likelihood scale (DDLS), and retinal nerve fiber layer (RNFL) asymmetry.
Results
Eighty-one consecutive patients from the Wills Eye Hospital glaucoma service were enrolled, 60 with glaucoma and 21 with ocular hypertension or glaucoma suspect. Thirty-one percent of subjects had MD asymmetry>5 dB, 19.7% had CDR asymmetry≥0.20, 26.7% had DDLS asymmetry≥2, and 38.2% had RNFL asymmetry>10 microns. Sensitivity values for pupillography were 93.3% (95% CI, 68.1–99.8) for detecting MD asymmetry, 80.0% (95% CI, 51.9–95.7) for CDR asymmetry, 100.0% (95% CI, 73.5–100.0) for DDLS asymmetry, and 69.2% (95% CI, 38.6–90.9) for RNFL asymmetry. Specificity values were 41.2% (95% CI, 24.7–59.3) for detecting MD asymmetry, 32.8% (95% CI, 21.3–46.0) for CDR asymmetry, 33.3% (95% CI, 18.0–51.8) for DDLS asymmetry, and 42.9% (95% CI, 21.8–66.0) for RNFL asymmetry. Pupillography amplitude score was correlated with MD asymmetry (r2=0.41, P<0.001) and area under the curve was 0.84.
Conclusion
Automated pupillography had higher sensitivity and lower specificity in detecting MD, CDR, DDLS, and RNFL asymmetry. Within the bounds of the cohort tested, this method had limited case-finding ability.
Similar content being viewed by others
Introduction
Relative afferent pupillary defect (RAPD) testing is an important technique in the evaluation of glaucoma. An RAPD can serve as an indicator of glaucomatous damage, particularly asymmetric damage.1 It is closely associated with other signs of glaucomatous damage including visual field changes,2 ophthalmoscopically apparent disc damage,3 and reduction of retinal nerve fiber layer (RNFL) thickness.4 An RAPD may even precede visual field changes.2
Historically, Galen of Pergamon was the first to perform crude RAPD testing in the 2nd century A.D. using a cover–uncover test, in which he employed an open window as a diffuse light source and alternately covered each eye while noting the pupillary response in the other.5 In current practice, RAPD testing is conventionally performed using the swinging flashlight method (SFM)—a technique initially described by Levatin6 in 1959 and later elaborated by Thompson.7, 8, 9 A positive SFM test suggests the presence of asymmetric damage involving the afferent limb of the light reflex pathway such as glaucoma-induced optic neuropathy.
Although it is possible to quantify the observed response using adjunct methods such as neutral density filters, the SFM is primarily a qualitative test.2 It relies upon visualizing no pupillary constriction or immediate or delayed pupillary dilation; if either of these responses is observed, an RAPD is believed to be present. The SFM is difficult to master and many potential sources of error may confound the results, including anisocoria, off-axis pupil illumination, and unequal retinal bleaching.10, 11 Pupil responses may be subtle and easily missed using the SFM. The magnifier-assisted SFM (MA-SFM), in which a +20 diopter lens is placed in front of the eyes during testing, may increase the sensitivity of RAPD detection in these subtle cases.12
SFM and MA-SFM do not measure important objective features of the pupillary response, such as the amplitude of constriction, latency of constriction, velocity of constriction, and duration of maximum constriction. These parameters, which are measurable by pupillography devices, allow examiners to precisely and objectively quantify the pupil response to light and serve as valuable indicators of glaucomatous damage.
The Konan RAPDx (Konan Medical, Irvine, CA, USA) is a patented pupillographic device. It uses a digital, high-definition, infrared camera with eye-tracking and automated blink detection technology. The RAPDx presents light stimuli to the eye and plots the pupil response curve as a graph. It also provides values to compare the amplitude of maximum constriction and latency of constriction onset between the two eyes.
Early identification of glaucomatous damage is essential in preventing disabling visual compromise and irreversible blindness. RAPD testing can offer valuable information regarding the presence of asymmetric disease and can aid in early diagnosis.13 A systematic meta-analysis has suggested that older generations of automated pupillography, including pupil cycle time, infrared video pupillography, and pupil perimetry have higher sensitivity and specificity than the SFM in detecting glaucoma.14 In this study, we directly compared the ability of the SFM, MA-SFM and the RAPDx pupillograph to detect asymmetric glaucomatous damage, as defined by standard clinical measures of optic nerve structure and function.
Materials and methods
This prospective cross-sectional study enrolled eligible patients at the Wills Eye Hospital in Philadelphia, PA, USA.
Patients were included if they had a diagnosis of any type of glaucoma, glaucoma suspect or ocular hypertension (intraocular pressure (IOP)>21 mm Hg). Exclusion criteria were abnormal ocular motility that prevents binocular fixation (eg, strabismus, nystagmus), any condition preventing adequate visualization and examination of the pupil or optic nerve (eg, dense corneal opacities or significant lens opacities), active infection or inflammation of the anterior or posterior segments of the eye, any intraocular surgical or laser procedure within the previous 4 weeks, and any other non-glaucomatous optic neuropathy that may cause an RAPD.
Clinical examination, visual field testing, and optical coherence tomography
The patients underwent clinical examinations to determine inclusion and exclusion criteria, best-corrected visual acuity, IOP by Goldmann applanation tonometry, vertical cup to disc ratio (CDR), and disc damage likelihood scale (DDLS) score. The DDLS, a measurement of the loss of neuroretinal rim tissue, is a 10-stage scale, with higher scores representing increasing optic disc damage. It has been shown to be correlated with visual field loss and has better interobserver reliability than CDR.15, 16 An inter-eye difference of DDLS≥2 or CDR≥0.20 were used as thresholds for asymmetry.
Octopus 900 standard automated perimetry (Haag-Streit, Koeniz, Switzerland) visual fields and Cirrus optical coherence tomography (Carl Zeiss Meditec, Dublin, CA, USA) performed within 6 months of RAPD testing were obtained from patients’ medical records. Inter-eye differences in visual field mean deviation (MD) and average RNFL thickness were calculated. An inter-eye difference of >5 dB was used as the threshold for MD asymmetry and a difference of >10 microns was used as the threshold for RNFL asymmetry.
SFM and MA-SFM
Three examiners performed SFM testing according to the methods described by Lankaranian et al3 and Kawasaki et al.17 A Finoff halogen transilluminator (Welch Allyn, Skaneateles Falls, NY, USA) at maximum light intensity (halogen HPX lamp with 3250 degrees K color temperature) was held 15 degrees below the visual axis for 3 s in the right eye, before being moved rapidly to the left eye for 3 s. The light was moved back to the right eye, and the cycle repeated 4–6 times until the result was confirmed. RAPD was indicated by immediate dilation of the pupil or no initial change followed by dilation that was definitely more marked than any dilation of the pupil of the other eye when the light was shined into that eye's pupil. All examiners were masked to the findings obtained by the other examiners; the test was considered positive if at least two examiners agreed. MA-SFM was performed in a similar fashion by two examiners, except a +20 diopter magnifying lens was placed in front of the eye being observed. This allows for better visualization of the pupil and has been shown to increase the sensitivity of RAPD detection.3 The test was considered positive if both examiners found an RAPD, or if one of the examiners found an RAPD which was corroborated by the third examiner’s SFM.
RAPD testing with the RAPDx pupillograph
Pupillary movements were recorded using the Konan RAPDx binocular infrared pupillograph. The device measures pupillary response to visual stimuli presented on a liquid crystal display and is capable of presenting stimuli in a variety of colors, intensities, and sizes. We used the standard setting in our testing: full-field, white light, and brightness of 70% of factory-set maximum intensity for 0.1-s stimuli, followed by 2-s inter-stimuli pauses.
For each pupil, the pupillograph plots the pupil response curve and calculates the amplitude of maximum constriction, which is the total change in pupil diameter divided by the pre-stimuli diameter, and latency of constriction, which is the number of milliseconds from stimulus onset until pupil velocity has reached 50% of the peak velocity of constriction.18 The pupillograph also calculates an RAPD score comparing the response of each pupil after stimulation of each eye:

where od and os is the mean response of both pupils after stimulation of the right and left eye, respectively.19 A positive value indicates a greater response to stimulation to the right eye compared with the left. In this study, we defined an RAPD as an amplitude score of >0.20 or <−0.20. A cut-off of 0.20 was chosen based on preliminary data from the manufacturer.
Statistical analysis
Data were collected and analyzed using SAS Statistical Software (SAS Institute, Cary, NC, USA). The sensitivity for each pupillary response measurement method (SFM, MA-SFM, pupillograph) was calculated for increasing levels of inter-eye differences in functional (MD) and structural (CDR, DDLS, and RNFL) measurements. Linear correlations and receiver operating characteristics (ROC) curves were created to determine the ability of the amplitude score to detect an asymmetry in MD, CDR, DDLS, and RNFL.
Satement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The Institutional Review Board approved the study protocol, and informed consent was obtained from all patients.
Results
Eighty-one patients were enrolled in this study. Forty-five subjects (56%) were female, and the mean age was 66.2±13.6 years. Sixty patients (74.1%) had a glaucoma diagnosis in at least one eye, whereas 21 (25.9%) had ocular hypertension or were glaucoma suspects. The demographics and clinical characteristics of the subjects are listed in Table 1. MD asymmetry>5 dB was noted in 15/49 (30.6%) subjects, CDR asymmetry≥0.20 in 15/76 (19.7%) subjects, DDLS asymmetry≥2 in 12/45 (26.7%) subjects, and RNFL asymmetry>10 microns in 13/34 (38.2%) subjects.
SFM and MA-SFM detected 12/57 (21.1%) and 20/57 (35.1%) of the RAPDs detected by the pupillograph (Table 2), respetively. SFM and MA-SFM detected 2/24 (8.3%) and 7/24 (25.9%) RAPDs that the pupillograph detected as having amplitude scores of <0.20. There was fair inter-rater reliability between the SFM (κ=0.26) and MA-SFM examiners (κ=0.30).
Table 3 describes the sensitivity and specificity of each method in relation to inter-eye differences in MD, CDR, DDLS, and RNFL. For each variable, the pupillograph had higher sensitivities for clinically significant cut points. MA-SFM consistently had the next highest sensitivities, followed by SFM. The sensitivity of the pupillograph was 93.3% (95% CI, 68.1–99.8) for MD asymmetry>5 dB, whereas MA-SFM and SFM sensitivities were 66.7% (95% CI, 38.4–88.2) and 33.3% (95% CI, 11.8–61.6), respectively. Similarly, pupillograph sensitivity was 80.0% (95% CI, 51.9–95.7) for a CDR asymmetry of ≥0.20, whereas MA-SFM and SFM sensitivities were 60.0% (95% CI, 32.3–83.7) and 40.0% (95% CI, 16.3–67.7), respectively. The sensitivity of the pupillograph was 100% (95% CI, 73.5–100.0) for detecting a DDLS asymmetry of ≥2, and 69.2% (95% CI, 38.6–90.9) for detecting RNFL asymmetry of >10 microns. For each variable, the pupillograph had lower specificities for the same cut points, compared with SFM and MA-SFM.
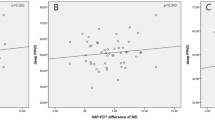
As depicted in Figure 1, pupillograph amplitude scores were correlated with both structural and functional measures of disease severity. Correlations were significant for MD (r2=0.41, P<0.001) and RNFL (r2=0.27, P<0.005). ROC curves, showing the ability of pupillograph amplitude score to discriminate clinically significant values of inter-eye asymmetry, are shown in Figure 2. The area under the curve (AUC) value for detecting MD asymmetry of >5 dB was 0.84, indicating that this device had a moderate ability to make this discrimination. AUC values for detecting CDR asymmetry of ≥0.20 was 0.62, DDLS asymmetry of ≥2 was 0.81, and RNFL asymmetry of >10 microns was 0.57. Disease severity, indicated by mean MD of the two eyes, was not correlated with amplitude score (r2=0.04, P=0.16).
Discussion
The RAPD has long been recognized as a characteristic finding in glaucoma and can even precede visual field loss.13 The results of the study showed that pupillography had the highest sensitivity and lowest specificity in using the RAPD to detect asymmetry in patients with glaucoma, followed by MA-SFM and SFM.
The RAPD is intrinsically a binocular test that depends on asymmetric damage, providing a comparison of the damage in the afferent pathway of each eye. As there is currently no gold standard to determine the absolute amount of damage to each optic nerve, we used functional and structural measures of glaucomatous optic neuropathy to estimate afferent pathway damage for each eye and determine the level of asymmetry. In our sample, a similar number of subjects met our criteria for asymmetry: MD>5 dB (30.6%), CDR≥0.20 (19.7%), DDLS≥2 (26.7%), or RNFL>10 microns (38.2%). These subjects were used as the baseline for asymmetric damage.
Prior studies have shown that an RAPD can be detected with the SFM in 30–70% of patients with gluacoma.20, 21 In this study, the SFM detected an RAPD in 17.3% of patients. In comparison, the MA-SFM detected about twice as many RAPDs (33.3%) as the SFM. This confirms prior reports that using a +20 diopter lens in the MA-SFM increases the sensitivity of the SFM to detect RAPDs.3 Using MD, CDR, DDLS, and RNFL measurements to quantify the amount of glaucomatous damage, the MA-SFM detected more subtle functional and structural damage compared with the SFM.
The pupillograph, a quantitative test for measuring pupillary response, outperformed both manual methods of SFM and MA-SFM in detecting inter-eye differences in MD, CDR, DDLS, and RNFL. At a threshold of MD asymmetry>5 dB, pupillography sensitivity was 93.3%. However, there was a slight drop in sensitivity above this threshold, likely due to variability from the low number of subjects between 5 and 10 dB MD asymmetry. Pupillography sensitivity values reached 100% in detecting patients who had inter-eye differences in MD >10 dB (12.2% of subjects), CDR≥0.4 (3.9% of subjects), and DDLS score≥2 (26.7% of subjects). The larger number of subjects included in the DDLS asymmetry≥2 group compared with the CDR asymmetry≥0.4 group highlights the higher sensitivity of DDLS for detecting ophthalmoscopically apparent glaucomatous damage.22
We found moderate correlations between the amplitude score of the pupillograph and MD (r2=0.41) and RNFL (r2=0.27) asymmetry. These correlations indicate that the sensitivity of pupillography increases as the disease becomes more asymmetrical, with other authors reporting similar r2 values (0.43–0.59).18, 19 Reports using other pupillographic machines have demonstrated stronger correlations (r2=0.73–0.83) between the RAPD and MD asymmetry.23, 24 Reported correlations likely differ due to variations in the pupillography device, testing parameters, and study populations. Other studies have investigated various pupillograph parameters to increase its sensitivity. Tatham et al18 examined glaucoma patients with the Konan RAPDx and reported a similar moderate correlation between MD and pupillographic asymmetry when using bright white stimuli (r2=0.43), compared with a low correlation when using colored stimuli (r2=0.18). However, they also found that a dim peripheral stimuli was the best for discriminating glaucoma patients from controls.18
Using an amplitude score of 0.2, the specificity of the pupillograph for detecting asymmetry in MD, CDR, DDLS, and RNFL was lower compared with SFM and MA-SFM. This suggests that this cut-off point is either overly sensitive or detects the presence of asymmetry before other structural or functional damage is noted.
We reported an AUC of 0.84 for detecting a 5 dB MD asymmetry using the pupillograph and an AUC of 0.57 for a 10-micron RNFL asymmetry. Tatham et al18 reported AUCs of 0.58, 0.71, 0.82, and 0.90 for detecting patients with 0, 5, 10, and 15 dB MD asymmetry, respectively, which is similar to the AUC of 0.84 we reported for a 5 dB MD asymmetry. This trend indicates that the two tests are in strongest agreement when the defect in asymmetry is large. They concluded that pupillography has poor performance in symmetric disease.18 In contrast, Kalaboukhova et al25 reported an AUC of 0.92 when comparing glaucomatous patients with controls using a custom-built setup that measured pupil area instead of diameter. Others have demonstrated that using a model including age and asymmetry in pupillary contraction amplitude, velocity, and latency can increase the sensitivity and specificity of pupillography.26 Although these testing parameters were not explored in this study, further investigation with varying parameters can likely improve the sensitivity of this device in detecting glaucomatous damage in symmetric or slightly asymmetric disease.18
Early detection of glaucoma through effective screening is an important part of preserving visual function and reducing preventable blindness around the world. In an evaluation of various glaucoma screening methods in Africa, CDR≥0.7 combined with an RAPD gave the optimum sensitivity and specificy.27 In our study, the MA-SFM and pupillography had higher sensitivity and lower specificity than the SFM and further studies should be conducted to determine their feasibility and applicability in glaucoma screening.
The current study has several limitations. First, only a relatively small number of patients showed asymmetry based on our inclusion criteria. The analysis was also limited by the number of patients who had missing MD, DDLS, or RNFL data. However, the proportion of patients with asymmetric glaucoma was similar to other reports.14 Second, we had a heterogeneous sample of patients that included subjects with diagnosis of glaucoma, glaucoma suspect, and ocular hypertension. Patients with glaucoma suspect and ocular hypertension status may have less asymmetric damage than those with more advanced disease, and the extent of this asymmetry may vary according to the clinical index used. Finally, it is difficult to assess the true degree of glaucomatous asymmetry based on MD, CDR, DDLS, or RNFL as these indicators are only surrogates of true disease severity. In the absence of a gold standard marker of neuronal dysfunction in glaucoma, agreement among existing clinical assessments must be the initial benchmark for novel diagnostic modalities.
In conclusion, this study showed that pupillography is more sensitive than MA-SFM, which is more sensitive than SFM, in detecting asymmetric damage related to glaucoma. Specificity was lower using pupillography compared with SFM and MA-SFM. We have demonstrated a correlation between the inter-eye pupillary response and traditional measures of functional and structural asymmetry. Within the bounds of the cohort tested, automated pupillography had limited case-finding ability.

References
Kaback MB, Burde RM, Becker B . Relative afferent pupillary defect in glaucoma. Am J Ophthalmol 1976; 81: 462–468.
Johnson LN, Hill RA, Bartholomew MJ . Correlation of afferent pupillary defect with visual field loss on automated perimetry. Ophthalmology 1988; 95: 1649–1655.
Lankaranian D, Altangerel U, Spaeth GL, Leavitt JA, Steinmann WC . The usefulness of a new method of testing for a relative afferent pupillary defect in patients with ocular hypertension and glaucoma. Trans Am Ophthalmol Soc 2005; 103: 200–207; discussion 207-208.
Tatsumi Y, Nakamura M, Fujioka M, Nakanishi Y, Kusuhara A, Maeda H et al. Quantification of retinal nerve fiber layer thickness reduction associated with a relative afferent pupillary defect in asymmetric glaucoma. Br J Ophthalmol 2007; 91: 633–637.
Thompson HS, Corbett JJ . Asymmetry of pupillomotor input. Eye 1991; 5: 36–39.
Levatin P . Pupillary escape in disease of the retina or optic nerve. Arch Ophthalmol 1959; 62: 768–779.
Thompson HS . Afferent pupillary defects. Pupillary findings associated with defects of the afferent arm of the pupillary light reflex arc. Am J Ophthalmol 1966; 62: 860–873.
Thompson HS . Pupillary signs in the diagnosis of optic nerve disease. Trans Ophthalmol Soc UK 1976; 96: 377–381.
Thompson HS, Corbett JJ, Cox TA . How to measure the relative afferent pupillary defect. Surv Ophthalmol 1981; 26: 39–42.
Bremner FD . Pupil assessment in optic nerve disorders. Eye 2004; 18: 1175–1181.
Kardon R . Pupillary light reflex. Curr Opin Ophthalmol 1995; 6: 20–26.
Ichhpujani P, Rome JE, Jindal A, Khator P, Leiby BE, Gordon H et al. Comparative study of 3 techniques to detect a relative afferent pupillary defect. J Glaucoma 2011; 20: 535–539.
Kohn AN, Moss AP, Podos SM . Relative afferent pupillary defects in glaucoma without characteristic field loss. Arch Ophthalmol 1979; 97: 294–296.
Chang DS, Xu L, Boland MV, Friedman DS . Accuracy of pupil assessment for the detection of glaucoma: a systematic review and meta-analysis. Ophthalmology 2013; 120: 2217–2225.
Henderer JD, Liu C, Kesen M, Altangerel U, Bayer A, Steinmann WC et al. Reliability of the disk damage likelihood scale. Am J Ophthalmol 2003; 135: 44–48.
Bayer A, Harasymowycz P, Henderer JD, Steinmann WG, Spaeth GL . Validity of a new disk grading scale for estimating glaucomatous damage: correlation with visual field damage. Am J Ophthalmol 2002; 133: 758–763.
Kawasaki A, Moore P, Kardon RH . Variability of the relative afferent pupillary defect. Am J Ophthalmol 1995; 120: 622–633.
Tatham AJ, Meira-Freitas D, Weinreb RN, Zangwill LM, Medeiros FA . Detecting glaucoma using automated pupillography. Ophthalmology 2014; 121: 1185–1193.
Sarezky D, Krupin T, Cohen A, Stewart CW, Volpe NJ, Tanna AP . Correlation between intereye difference in visual field mean deviation values and relative afferent pupillary response as measured by an automated pupillometer in subjects with glaucoma. J Glaucoma 2014; 23: 419–423.
Skorkovska K, Wilhelm H, Ludtke H, Wilhelm B . Relative afferent pupillary defect in glaucoma. Klinische Monatsblatter fur Augenheilkunde 2011; 228: 979–983.
Charalel RA, Lin HS, Singh K . Glaucoma screening using relative afferent pupillary defect. J Glaucoma 2014; 23: 169–173.
Danesh-Meyer HV, Gaskin BJ, Jayusundera T, Donaldson M, Gamble GD . Comparison of disc damage likelihood scale, cup to disc ratio, and Heidelberg retina tomograph in the diagnosis of glaucoma. Br J Ophthalmol 2006; 90: 437–441.
Schiefer U, Dietzsch J, Dietz K, Wilhelm B, Bruckmann A, Wilhelm H et al. Associating the magnitude of relative afferent pupillary defect (RAPD) with visual field indices in glaucoma patients. Br J Ophthalmol 2012; 96: 629–633.
Chang DS, Boland MV, Arora KS, Supakontanasan W, Chen BB, Friedman DS . Symmetry of the pupillary light reflex and its relationship to retinal nerve fiber layer thickness and visual field defect. Invest Ophthalmol Vis Sci 2013; 54: 5596–5601.
Kalaboukhova L, Fridhammar V, Lindblom B . Relative afferent pupillary defect in glaucoma: a pupillometric study. Acta Ophthalmol Scand 2007; 85: 519–525.
Chang DS, Arora KS, Boland MV, Supakontanasan W, Friedman DS . Development and validation of an associative model for the detection of glaucoma using pupillography. Am J Ophthalmol 2013; 156: 1285–1296 e1282.
Cook C, Cockburn N, van der Merwe J, Ehrlich R . Cataract and glaucoma case detection for Vision 2020 programs in Africa: an evaluation of 6 possible screening tests. J Glaucoma 2009; 18: 557–562.
Acknowledgements
We acknowledge Lalita Gupta, Camila Zangalli, Alice Zhang, Eileen Wang, and Michael Pro for their help with patient recruitment and testing, data collection, and clinical examinations. The Glaucoma Service Foundation to Prevent Blindness provided funding to support this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Waisbourd, M., Lee, B., Ali, M. et al. Detection of asymmetric glaucomatous damage using automated pupillography, the swinging flashlight method and the magnified-assisted swinging flashlight method. Eye 29, 1321–1328 (2015). https://doi.org/10.1038/eye.2015.106
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.106
This article is cited by
-
Pupillary response to chromatic light stimuli as a possible biomarker at the early stage of glaucoma: a review
International Ophthalmology (2022)
-
Significant correlations between photopic negative response, afferent pupillary defect, and mean defects of visual fields in asymmetric optic nerve disorders
Graefe's Archive for Clinical and Experimental Ophthalmology (2020)