Abstract
Purpose
To characterise the history, clinical and histopathological features of patients with bilateral nasal and temporal peripheral hypertrophic subepithelial corneal degeneration in a German population.
Methods
A detailed ophthalmological and dermatological history and clinical findings were recorded of nine patients with bilateral simultaneous nasal and temporal peripheral corneal degeneration from two centers in Germany. Excised tissues were studied by histopathology, immunohistochemistry, and transmission electron microscopy.
Results
Foreign body sensation and need of artificial tear substitutes were the only symptoms reported regularly. Schirmer’s and Jones-test were normal in all, but fluorescein break-up time of >10 s was found in five eyes of four patients. Best corrected visual acuity was reduced only under glare conditions. Corneal topography revealed irregular astigmatism in 13 of 14 eyes. Follow-up median time was 35 months. Most cases were stable within the follow-up period. Light and electron microscopy revealed the findings of superficial vascularised corneal hypertrophic scars, oxytatlan fibers, and discontinued Bowmans layer.
Conclusion
In this series of German patients with peripheral hypertrophic subepithelial corneal degeneration, the changes were predominantly located in the palpebral aperture and often present in both eyes. No associated surface disease could be established in this study. Light and transmission electron microscopy showed histological features that are similar to Salzmann’s corneal changes without any inflammation. We hypothesise that light exposure and a localised limbal insufficiency could be involved in the pathogenesis.
Similar content being viewed by others
Introduction
Salzmann’s nodules (SN) are subepithelial, elevated bluish-white corneal opacities of non-inflammatory origin, with a specific peripheral circular pattern.1, 2, 3, 4, 5, 6, 7 What has been termed Salzmann’s degeneration is predominantly unilateral, presenting at any time in life with a female preponderance. Although the pathophysiology remains undefined until present, chronic ocular surface irritation or inflammation are thought to be the initiating conditions. The corneal nodules can be associated with a peripheral pannus. More common differential diagnosis of such prominent subepithelial nodules associated with peripheral corneal vascularisation include posttraumatic scars and pterygium. Less well known are two entities that present with bilateral peripheral changes similar to a pterygium, which were classified as peripheral hypertrophic subepithelial corneal degeneration (PHSCD) and autosomal dominant pterygoid corneal dystrophy, which clinically show also features of SN.
Between 2003 and 2011, we observed a pattern of peripheral pterygium-like corneal changes in nine patients that showed none of the conjunctival changes of a typical pterygium, but included Salzmann’s like corneal opacities. Strikingly these were all bilateral as well as both nasal and temporal. None of the previous descriptions in literature showed these high numbers of strict nasal and temporal affections. We performed a thorough examination of the ocular surface of these patients with this distinct corneal pathology. Here, we describe the typical clinical and microscopic findings of what may be considered a subtype of PHSCD with a bitemporal and binasal pattern.
Subjects and methods
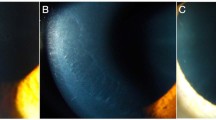
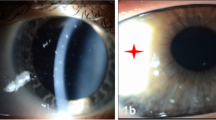
Between 2003 and 2011 we examined nine patients (seven female, two male; median age 43 years (29–55, ±10 years) at two centers (University Eye Hospital Wuerzburg, Germany and University Eye Hospital Luebeck, Germany) who uniformly presented with a bilateral, nasal and temporal superficial pannus, that is, intra- or subepithelial neovascularisation and scar tissue, in the palpebral aperture (Figure 1). This extended over approximately two limbal clock hours. Central to the pannus, elevated white subepithelial opacities similar to SN were located. Median follow-up period was 35 months (range 20–82 months), only one patient showed progression of changes after excision.
The medical history and clinical findings of all patients were recorded following a questionnaire and protocol. This included the family history, systemic and ophthalmic history of diseases, drug use, known allergies, and any signs of ocular disease. We specifically searched for any signs and symptoms of dermatological disorders. The ophthalmic history assessed any previous trauma, including eye surgery, use of eye drops, and contact lenses. Best corrected spectacle visual acuity (BCVA) using decimal charts in 5 m distance and brightness acuity test were recorded.
A full clinical ophthalmic examination was performed including measurement of the vertical and horizontal palpebral aperture, exophthalmometry, corneal topography, aesthesiometry, Fluoresceine break-up time, Schirmer test with and without anaesthesia over 5 min and syringing of the lacrimal drainage system. In addition all patients were assessed by a dermatologist.
In three patients the superficial corneal opacification and pannus were easily stripped off the underlying corneal surface by manual superficial keratectomy and processed for light and electron microscopy. For electron microscopy, specimens were fixed in 4% paraformaldehyde/0.1% glutaraldehyde in 0.1-M phosphate buffer, postfixed in 2% buffered osmium tetroxide, dehydrated in graded alcohol concentrations, and embedded in epoxy resin. Semithin sections (1 μm) were stained with toluidine blue, ultrathin sections were stained with uranyl acetate-lead citrate and examined with a transmission electron microscope (TEM) (EM 906E; Zeiss, Oberkochen, Germany).
For light microscopy, specimens were fixed in 4% paraformaldehyde/0.1% glutaraldehyde in 0.1 M phosphate buffer and embedded in paraffin according to standard protocols. 5-μm thick paraffin sections were stained with periodic acid-Schiff (PAS), hematoxylin-eosin, Masson trichrome stain, van Gieson’s stain for elastin, Congo red for amyloid, and acid mucopolysaccharides (AMP). Immunohistochemistry was performed by the peroxidase-labeled streptavidin-biotin method, using the LSAB Plus-kit according to the manufacturer‘s instructions (DAKO, Glostrup, Denmark). Briefly, sections were incubated with primary antibody dilutions, biotinylated link antibody, and peroxidase-conjugated streptavidin for 30 min each. As a chromogenic substrate, 3-Amino-9-ethyl carbazole was used, and Mayer‘s hemalum was used as a counter stain. Monoclonal antibodies against pan-cytokeratin (clone 80, Abcam, Cambridge, UK), corneal epithelial cytokeratin 3/12 (clone AE5, Merck-Millipore, Darmstadt, Germany), cytokeratin 10 (clone DE-K10, Abcam), cytokeratin 13 (clone KS-1A3, Novocastra, Newcastle upon Tyne, UK), cytokeratin 19 (clone BA17, Merck-Millipore), fibrillin-1 (clone 26, Merck-Millipore), vimentin (clone V9, DAKO), and alpha-smooth muscle actin (clone 1A4, Sigma, St Louis, MO, USA) were used at concentrations of 1:50−1:100. In negative control experiments, the primary antibody was omitted or replaced by an irrelevant primary antibody.
Results
History
The family, general and drug histories were unremarkable in all patients. Two reported a history of seasonal allergies. Two patients had occasionally used soft contact lenses until several years before consultation.
Symptoms and signs
All except one patient suffered of foreign body symptoms and applied topical artificial lubricants regularly two to six times a day. Automated refraction revealed an against the rule astigmatism in 15 of 16 eyes (median=−2.0 D, min −0.25 D to −10.75 D max). Corneal topography confirmed the irregularity of the astigmatism. Median spectacle best corrected decimal visual acuity was 0.9 (min 0.2–1.25 max). Two patients showed reduced brightness acuity well below the BCVA under room light conditions (Table 1).
In all patients, slitlamp biomicroscopy revealed a bilateral nasal and temporal pannus with vascularisation extending over one to three limbal clock hours almost exclusively in the palpebral apperture. Central to the pannus, elevated subepithelial white stromal opacities were located. The detailed dimensions of the lesions and the maximal vascularisation towards the center of the cornea are shown in Table 1. All eyes presented with normal limbal corneal findings and a clear cornea at six and 12 o’clock. Six eyes of four patients had distorted conjunctival vessels and a mild conjunctival thickening adjacent to the corneal changes, while the remaining eyes were normal in this respect. Lower and upper palpebral conjunctiva were completely normal.
Schirmer (median 25 mm, 20–25 mm) and Jones-test (median 20 mm, 15–25 mm) were normal in all eyes. Fluorescein break-up time was reduced in four patients/five eyes to less than 10 s and the break-up specifically occurred in areas with surface irregularities. Aesthesiometry showed minimally reduced corneal sensation in two patients/three eyes (median 0.4 g/mm2, min 0.4 to 0.7 g/mm2 max).
Exophthalmometry readings indicated a normal range of measurements (median 13 mm, min 11–17 mm max). Vertical (median 8.5 mm, min 7–12 mm max) as well as horizontal palpebral aperture (median 25.5 mm, min 25–27 mm max) were equally unremarkable. Lacrimal drainage and all intraocular findings were normal.
All except one patient showed no progression of the corneal changes within the median follow-up period of 35 months (range 20–82 months) after surgery or initial visit.
Case report
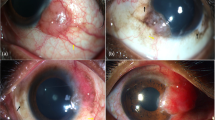
A 27-year-old myopic female patient had experienced intermittent symptoms of itching, dryness, and foreign body sensation for 7 years. Three years prior to our consultation, her ophthalmologist had noticed peripheral corneal scars. On presentation in our department the patient complained of photophobia and cosmetically disturbing bilateral peripheral corneal changes in the palpebral aperture. She had worn soft contact lenses during sports for two years, but had discontinued using them because of increasing discomfort. She was using unpreserved lubricants three-to four-times daily.
Best spectacle corrected visual acuity was 1.0 in both eyes (OD −1.0 D–1.0 D/9°; OS −1.5 D sph.). Slitlamp examination showed bilateral peripheral pannus and central to this superficial white opacifications in the nasal and temporal cornea leaving a minimum clear central area of 6.7 mm. The bulbar conjunctiva was completely normal, but a mild subtarsal papillary conjunctivits was present in both eyes. A dermatological assessment revealed no skin alterations and no signs of allergy.
During the following 12 months the pannus progressed in her RE and she developed an irregular astigmatism of 3 D (Ksteep=7.37/100° and Kflat 8.17/10°) and her BCVA decreased to 0.5. In May 2005, the superficial corneal changes were removed by manual keratectomy. Although the pannus recurred in the peripheral 2.0 mm of the cornea, astigmatism was reduced to 0.25 D and BCVA increased to 1.0. Slit lamp microscopical changes and visual acuity remained unchanged until final follow-up in March 2011 (Figure 2).
Histopathology
Histopathologic evaluation of the superficial corneal opacification and pannus tissue was performed in three patients. Light microscopy showed a multilayered, non-keratinised squamous epithelium of varying thickness with slightly edematous basal cells and without any goblet cells. The epithelial basement membrane was irregular and discontinuous and Bowman’s layer was fragmented or absent (Figure 3). The subepithelial stroma presented as a loose vascularised hypocellular pannus tissue adjoining a rather dense collagenous hypercellular scar tissue (Figures 3a–d). The pannus zone revealed numerous blood vessels, few fibroblasts, and elastoid degeneration of collagen fibers (Figures 3a and c). In contrast, the opacification zone displayed abundant activated fibroblasts, an irregular arrangement of densely packed collagen lamellae interspersed with hyalinised inclusions and few blood vessels like the advancing head of a pterygia (Figures 3b, d and ). Masson’s trichrome stain demonstrated elastoid degeneration of the collagen fibers in the pannus area (Figures 3e and f) and densely packed collagen lamellae in the scar area. AMP staining detected minor deposits of acid mucoplysaccharides, whereas Congo red staining did not detect any amyloid deposits (not shown). Unlike pterygium no signs of active inflammation or goblet cell colonisation was observed.
Light microscopic appearance of the corneal lesion of patient 1 (134/08). (a) Region of vascularised hypocellular pannus tissue (PAS staining, × 100). (b) Transition zone between vascularised hypocellular pannus tissue (right) and dense hypercellular scar tissue (left) (PAS staining, magnification × 100). (c) Detail of pannus tissue showing elastoid degeneration of collagen fibers (PAS staining, magnification × 200). (d) Detail of scar tissue showing irregularly arranged, densely packed collagen fibers interspersed with abundant activated fibroblasts (PAS staining, magnification × 200). (e, f) Masson’s trichrome stain shows few regular collagen fibers (blue) but large areas of elastoid degeneration (purple, arrows) and hyaline degeneration (asterisks) of collagen fibrils (magnification × 100 in (e), × 200 in (f)). A full color version of this figure is available at the Eye journal online.
Transmission electron microscopic appearance of the corneal lesion of patient 1 (342/08). (a) Overview of the corneal epithelium (EP) with edematous basal cells overlying Bowman’s layer (BL) and a disorganised stroma (ST) containing disrupted collagen lamellae and abundant areas of electron-lucent substances. (b). Along the basal aspect of the epithelium (EP), fragments of basement membrane (closed arrow) and bundles of anchoring fibrils (open arrows) can be observed together with a abnormally structured Bowman’s layer (BL). (c) Subjacent to Bowman’s layer (BL), hyalinised inclusion bodies (HB) traversed by bundles of microfibrils are focally present. (d) The subepithelial stroma consists of disrupted collagen lamellae (CO), electron-lucent areas (asterisk), and activated keratocytes (KC). (e) Detail of oxytalan microfibrils (asterisks). (f) Subepithelial blood vessel (BV).
TEM of the opacification zone (Figure 4), performed in the samples of three patients, confirmed focal thickenings and interruptions of the epithelial basement membrane, disorganised bundles of subepithelial anchoring fibrils as well as an abnormal structure of Bowman’s layer, containing cellular debris and scattered plaques of remaining basement membrane material (Figures 4a and b). In the subepithelial stroma, irregularly arranged collagen fibers were interspersed with prominent accumulations of an electron-lucent material probably representing glycosaminoglycans, globular inclusions of a hyalinised amorphous material, and bundles of 10–12 nm microfibrils resembling oxytalan fibrils (Figures 4c–e). Most of the abundant stromal keratocytes appeared metabolically active as indicated by dilated cisterns of rough endoplasmic reticulum. Occasionally, small blood vessels were observed subepithelially (Figure 4f).
By immunohistochemical evaluation, the epithelial cells showed prominent staining for pan-cytokeratin (Figure 5a), and partial staining for cytokeratin 3/12, particularly in the scarred regions (Figure 5b), and cytokeratins 13 and 19, particularly in the pannus regions (Figure 5c). Cytokeratin 10, an epidermis-specific marker, was negative in epithelial cells (Figure 5d). The subepithelial stroma showed a pronounced immunoreactivity for fibrillin-1 indicating the presence of oxytalan fibrils specific for SN (Figure 5e). The stromal cells were positive for vimentin and alpha-smooth muscle actin indicating transformation in myofibroblasts (Figure 5f).
Discussion
We report on 18 eyes of nine, predominantly female patients with bilateral, nasal and temporal subepithelial keloid-like corneal changes consisting of a peripheral fibrovascular pannus and centrally adjacent superficial dense white scars, with little or no conjunctival changes. This entity is most likely a subform of previous described PHSCD with corneal changes involving the nasal and temporal corneas of both eyes, but not other areas of the cornea.5, 8, 9 The disorder in our patients became symptomatic in the fourth and fifth decade of life and its biomicroscopical corneal appearance and geographic pattern were pterygium-like with the whitish opacifications being the dominant clinical finding resembling SN. All except one patient showed no changes within the follow-up time of a mean of 35 months.
Several aspects, however, suggest that our patients were not affected by simple pterygia. This long known entity has been described as a triangular encroachment of the bulbar conjunctiva onto the cornea, which is always localised in the palpebral aperture—predominantly on the nasal side - and always includes conjunctival changes, which were absent or only minimal in our patients. The pterygium head—unlike in our cases—is usually firmly attached to the underlying stroma. Also simultaneous nasal and temporal pterygia– are rare, with a published prevalence of ∼2.4% of all pterygium cases and bilateral simultaneous nasal and temporal involvement in caucasians, as observed in our patients, is even rarer.10 Near the equator bilateral pterygia are seen more often supposedly because of higher light exposition of these patients, but this is extremely rare in central Europe. Dolezalova10 reported a bilateral nasal and temporal pterygium in only one of 1388 examined patients with pterygia.
The white superficial prominent corneal changes we observed are similar to SN, which also show a female preponderance. However, our patients were on average younger and the geographic distribution of corneal opacities different from SN in that they were always bilateral and almost exclusively limited to the palpebral aperture.11 Although such a pattern has been described for SN observed myopes with an extensive history of contact lens wear, only two of our patients had ‘occasionally’ used contact lenses years prior to initiation of symptoms.11, 12
The histopathologic findings in our patients were similar to those of pterygoid corneal dystrophy with a discontinuous basement membrane, absence/fragmentation of Bowman’s layer, subepithelial fibrovascular tisse, elastoid degeneration of collagen and elastic fibers, subepithelial hyalinisation of connective tissue, and epithelial cells expressing both cornea- (K12) and conjunctiva-specific markers (K13).7 This clinically also presents as a bilateral corneal opacification with nasal/temporal involvement and multiple relapses after pterygium surgery at young age and reduced visual acuity because of high irregular astigmatism. This was thought to be associated with an autosomal dominant history of family involvement. However, the family history of our patients was completely normal.
Our specimens showed features associated with SN or the head of a pterygium including pannus formation, irregular thinned epithelium, absence/fragmentation of Bowman’s layer, hypercellular scar tissue, hyaline deposits, oxytalan fibrils, myofibroblasts, and conjunctiva-specific markers (K19) in the epithelium, as well as vimentin and alpha-SMA-positiviy in stromal cells.13, 14 More specifically SN are histologically characterised by oxytalan fibres, which are absent in pterygia.13 Fibrillin-1 staining and TEM revealed oxytalan fibrils in the excisional biopsies taken from our patients, suggesting that histologically these were not pterygia but SN.
In as recent review on Salzmann’s nodular degeneration, Sundmacher15—also based in Germany— stated that most cases in his experience never had a history or signs of conjunctival or corneal inflammation and were often also bilateral. Hence, contrary to frequent text book reports, he suggested to considers this to of dystrophic and not degenerative origin. Graue-Hernandez et al described 108 patients with 180 eyes with Salzmann nodular degeneration.16 They concluded that this entity remains of uncertain aetiology in which inflammation may have a role. The new international classification on corneal dystrophies therefore does not classify SN to any group of corneal dystrophies.17 Lack of family history as well as no recurrence or disease progression in two of three of our patients with excision of the corneal changes points more to a kind of corneal degeneration even if there are no proven signs of corneal inflammation in ophthalmic history. However this question may only be decided following genetic analysis.
Woelter-Roessler et al7 described a family with three members with bilateral temporal and nasal pterygia without special dust or light exposure. All three patients suffered of multiple relapses after pterygium surgery at young age and reduced visual acuity because of high irregular astigmatism. The authors classified these changes as inherited pterygiod corneal dystrophy with putative autosomal dominant heredity. As previously described none of our patients reported similar cases of corneal changes or loss of vision in early age in any other family member.
Maust and Raber5 described six cases of bilateral PHSCD which they considered to be a variant of Salzmann’s nodular degeneration. Equally, all their patients were female, two had a history of systemic diseases (sarcoidosis and thyroid disease), three used contact lenses. Five patients showed bilateral peripheral corneal opacities similar to our patients. However, their patients presented only with changes in the nasal or temporal sector. Only one patient showed bilateral nasal and temporal corneal changes. The authors also reported a mild conjunctival injection in three patients and suggested a chronic low-grade inflammation as cause of the corneal changes.
Gore et al8 recently published a series of 22 patients (20 women, 2 men), median age 42 years, which he also categorised to PHSCD. Only 3 of these 22 patients showed bilateral temporal and nasal corneal changes. Histopathological examination in seven cases revealed similar subepithelial fibrosis; no significant inflammatory cells could be shown. One of the bilateral, bitemporal and binasal cases showed also oxytalan fibres as we could identify in all of our histopathological examined tissues. Most of our patients also had no history of ocular inflammation. Our patients may represent a more extreme form of the variant of the entity described by Maust et al and Gore et al with little or no concomitant conjunctival alterations.
It remains unclear whether a localised limbal stem cell insufficiency is associated with the peripheral subepithelial hypertrophic corneal changed in our series, as is suggested by the superficial corneal pannus observed adjacent to the limbus. The bilateral, temporal and nasal corneal changes could have occurred simultaneously or sequentially. Although it remains speculative how long it took for these lesions to evolve the almost symmetrical presence of the corneal changes at first presentation points towards a simultaneous process. The predominant position in the palpebral apperture at 3 and 9 o’clock suggests that increased light exposure may be involved in the pathogenesis similar to pterygia, where it has been conceptualised into two stages: first, a progressive disruption of the limbal corneal-conjunctival epithelial barrier and second a progressive active ‘conjunctivalisation’ of the corneal surface.4 Increased ultraviolet light exposure of the ocular surface in the palpebral aperture is thought to be a major pathogenetic factor initiating these alterations.4 However, apart from two patients reporting extensive sunlight exposure during childhood, we found no clinical signs further supporting this theory, with exophthalmometry and palpebral aperture measures all being normal.
Progression of the corneal changes was associated with a hyperopic shift, probably because of an altered tear meniscus in the palpebral aperture. These hypertrophic corneal changes could easily be stripped off the underlying corneal surface by manual keratectomy. In extensive or recurrent cases after manual superficial keratectomy one could consider using adjunctive treatment with MMC or limbal stem cell autograft. However, if the findings would be associated with limbal insufficiency all further damage to the limbus should be avoided. Follow-up showed no progression except of one case after excision and one without excision. All other patients showed no progression over a median time of 35 months. In a previous study of PHSCD, progression of corneal changes occurred in 4 of 17 patients (median follow-up time 23 months (0–11 years)).8
In summary, we here present a group of predominant female patients with a special geographic pattern similar to previously described PHSCD. Light microscopy and TEM showed histological features that are similar to Salzmann’s corneal changes without any signs of inflammation. The process is bilateral and occurs frequently simultaneously in the nasal and temporal palpebral aperture suggesting light exposure as a contributing factor, although the pathophysiology remains unclear. The almost exclusive localisation in the palpebral fissure was not described in a larger number of patients in PHSCD before. Corneal changes seem to be stable over time; recurrences after surgical excision are rare. Ophthalmologists should be aware of this entity of corneal changes that - including this report – is now described in more than 35 cases in Germany and Great Britain.

References
Bock O, Mrowietz U . Keloids. A fibroproliferative disorder of unknown etiology. Hautarzt 2002; 53 (8): 515–523.
Bourcier T et al. Corneal keloid: clinical, ultrasonographic, and ultrastructural characteristics. J Cataract Refract Surg 2004; 30 (4): 921–924.
Cibis GW, Baudrimont M, Boutboul S, Thomas F, Borderie V, Laroche L . Corneal keloid in Lowe's syndrome. Arch Ophthalmol 1982; 100 (11): 1795–1799.
Kwok LS, Coroneo MT . A model for pterygium formation. Cornea 1994; 13 (3): 219–224.
Maust HA, Raber IM . Peripheral hypertrophic subepithelial corneal degeneration. Eye Contact Lens 2003; 29 (4): 266–269.
Rao SK, Fan DS, Pang CP, Li WW, Ng JS, Good WV et al. Bilateral congenital corneal keloids and anterior segment mesenchymal dysgenesis in a case of Rubinstein–Taybi syndrome. Cornea 2002; 21 (1): 126–130.
Wolter-Roessler E, Seitz B, Naumann GO . Pterygoid corneal dystrophy. Klin Monbl Augenheilkd 2002; 219 (9): 677–681.
Gore DM, Iovieno A, Connell BJ, Alexander R, Meligonis G, Dart JK . Peripheral hypertrophic subepithelial corneal degeneration: nomenclature, phenotypes, and long-term outcomes. Ophthalmology 2013; 120 (5): 892–898.
Jeng BH, Millstein ME . Reduction of hyperopia and astigmatism after superficial keratectomy of peripheral hypertrophic subepithelial corneal degeneration. Eye Contact Lens 2006; 32 (3): 153–156.
Dolezalova V . Is the occurrence of a temporal pterygium really so rare? Ophthalmologica 1977; 174 (2): 88–91.
Das S, Link B, Seitz B . Salzmann's nodular degeneration of the cornea: a review and case series. Cornea 2005; 24 (7): 772–777.
Wood TO . Salzmann's nodular degeneration. Cornea 1990; 9 (1): 17–22.
Obata H, Inoki T, Tsuru T . Identification of oxytalan fibers in Salzmann's nodular degeneration. Cornea 2006; 25 (5): 586–589.
Stone DU, Astley RA, Shaver RP, Chodosh J . Histopathology of Salzmann nodular corneal degeneration. Cornea 2008; 27 (2): 148–151.
Sundmacher R . Salzmann's nodular degeneration. Mostly an epithelial corneal dystrophy. Ophthalmologe 2012; 109 (4): 389–403.
Graue-Hernandez EO, Mannis MJ, Eliasieh K, Greasby TA, Beckett LA, Bradley JC et al. Salzmann nodular degeneration. Cornea 2010; 29 (3): 283–289.
Lisch W, Seitz B . New international classification of corneal dystrophies (CD). Ophthalmologe 2011; 108 (9): 883–896.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Schargus, M., Kusserow, C., Schlötzer-Schrehardt, U. et al. Peripheral hypertrophic subepithelial corneal degeneration presenting with bilateral nasal and temporal corneal changes. Eye 29, 88–97 (2015). https://doi.org/10.1038/eye.2014.236
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.236
This article is cited by
-
Chirurgische Abtragung von Salzmann-Knoten unter Anwendung von intraoperativem Mitomycin C
Der Ophthalmologe (2016)