Abstract
Purpose
To evaluate the long-term visual outcomes of pars plana vitrectomy (PPV) for polypoidal choroidal vasculopathy (PCV)-associated vitreous haemorrhage (VH).
Method
We retrospectively reviewed the records of patients with PCV-related VH who underwent PPV. The main outcome measures were best-corrected visual acuity (BCVA) and fundus findings at 3 months postoperatively and final visit.
Results
Seventeen eyes of 17 patients with massive subretinal haemorrhage (16.7±7.1 disc size of mean subretinal haemorrhage area) were enrolled. The mean postoperative follow-up period was 25.2 months. Four eyes received intravitreal bevacizumab injections, and three eyes underwent photodynamic therapy before the onset of VH. The mean BCVA improved from logarithm of the minimum angle of resolution (LogMAR) of 2.63±0.57 preoperatively to 1.43±0.82 at final visit (P<0.001). Among the eyes with initial polyps at subfoveal or juxtafoveal area, 16.70% achieved final BCVA ≥20/400 (LogMAR 1.3), whereas 87.50% of eyes with initial polyps at extrafoveal area had final BCVA ≥20/400 (Fisher’s exact test, P=0.026).
Conclusions
PCV with massive subretinal haemorrhage is at risk for breakthrough VH. The visual prognosis in eyes with PCV-related breakthrough VH is variable after vitrectomy. Initial polyps at the extrafoveal area led to better functional outcomes. Early vitrectomy may be beneficial for visual recovery after PCV-related VH.
Similar content being viewed by others
Introduction
Polypoidal choroidal vasculopathy (PCV) is characterized by orange-reddish polypoidal lesions beneath the retinal pigment epithelium (RPE) that usually lead to recurrent serous leakage and haemorrhage.1 PCV was previously described as ‘posterior uveal bleeding syndrome’2 and ‘multiple recurrent retinal pigment epithelial detachments in black women’3 more than two decades ago. The representative findings, indicated by indocyanine green angiography (ICG), are branching choroidal networks with terminal polypoidal dilatation of the choroidal vessels.4 PCV is more commonly found in Asian populations.5 However, the pathogenesis of PCV is still not fully understood.
PCV lesions tend to bleed easily, with a rate of 30–63.6% for subretinal haemorrhage6, 7, 8 and 4.5–19.9% for vitreous haemorrhage (VH).7, 8 In the clinical course of PCV, haemorrhage is usually acute, and visual acuity declines abruptly. Visual prognosis may be limited, with prolonged dense haemorrhages under the RPE or recurrent haemorrhages that lead to RPE and outer retina degeneration. Chan et al9 proposed a classification based on the clinical patterns at presentation, and each subgroup may have a different natural course or clinical outcome. The massive haemorrhagic group (group 4) was defined as a large subretinal or sub-RPE haemorrhage >2 Macular Photocoagulation Study disc areas in the macular region. In addition, breakthrough VH may also be encountered after treatments such as intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents,10 photodynamic therapy (PDT),11, 12 and pneumatic displacements.13 The visual prognosis of PCV with VH is variable and is generally considered to be poor.10, 12 However, in a case series reported by Jung et al,14 the visual outcome of vitrectomy for VH associated with PCV seemed to be better than that of the age-related macular degeneration (AMD) group. As there are few reports evaluating the outcomes of surgery for PCV-related VH,14, 15 we aimed to evaluate the long-term visual outcomes of PCV with breakthrough VH managed by early pars plana vitrectomy (PPV).
Materials and methods
We conducted a retrospective study of patients with PCV-related breakthrough VH undergoing PPV from August 2008 to March 2012 in the Department of Ophthalmology, National Taiwan University Hospital, Taipei, Taiwan. This study was approved by the Institutional Review Board of National Taiwan University Hospital, Taipei, Taiwan. The study complied with the guidelines of the Declaration of Helsinki. The collected preoperative and postoperative records included a complete medical and ophthalmic history, best-corrected visual acuity (BCVA) with Snellen chart, intraocular pressure, slit lamp examination, dilated fundus examination, optical coherence tomography, fundus fluorescein angiography (FFA), ICG, preoperative treatments, and operation records. Diagnoses of PCV were made preoperatively or postoperatively, based on the results of fundus examination, FFA, and ICG. A branching vascular network with polypoidal structures dilatation demonstrated by ICG was crucial for the confirmation of PCV. The locations of polypoidal lesions were classified into subfoveal, juxtafoveal (within 200 μm of the fovea), and extrafoveal.16
All patients underwent a standard 20-G system three-port PPV without subretinal manipulation. All operations were performed by a single retina surgeon (CH Yang). The intravitreal injection of bevacizumab was performed at the end of surgery if there was a fresh subretinal haemorrhage, serous retinal detachment (RD), serous RPE detachment, or cystoid macular oedema noted during operation. When an active PCV lesion with recurrent bleeding or leakage was found during the course of postoperative follow-up, an intravitreal bevacizumab injection was performed.
The main outcome measures were BCVA and fundus findings at 3 months postoperatively and at the final follow-up visit. Visual acuity values were converted into the logarithm of the minimal angle of resolution (LogMAR) for statistical analysis. Counting fingers (CFs) was converted to LogMAR 2.0, and hand motion (HM) was converted to LogMAR 3.0.17 Factors that might influence the visual outcome, including age, gender, presence of systemic disease, size of subretinal haemorrhage, duration of subretinal haemorrhage, duration of VH, location of polypoidal lesions, preoperative treatment with intravitreal bevacizumab injection, or PDT, were evaluated for correlation with the final functional outcomes by a logistic regression test. Fisher’s exact test was used to analyse categorical variables. Statistical analyses were conducted using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to be statistically significant.
Results
Seventeen eyes of 17 consecutive patients (9 men and 8 women) were included in this study. All cases were followed up for at least 6 months. The basic characteristics of these patients were summarized in Table 1. The mean age of the patients was 60.1±8.5 years (ranging from 45 to 81 years). The mean postoperative follow-up period was 25.18±20.23 months (ranged from 6 to 72 months). Six patients had hypertension, three patients had diabetes mellitus, and one patient was under anticoagulant use. Three patients (17.6%) had PCV lesions in both eyes.
Among these eyes with PCV-related VH, VH was the initial presentation in two eyes, and seven eyes developed spontaneous breakthrough VH during the follow-up period. Four eyes received intravitreal bevacizumab injections, and three eyes underwent PDT before the occurrence of breakthrough VH. The one remaining eye received pneumatic displacement for the subretinal haemorrhage, but RD and VH occurred 10 days later. The average duration from subretinal haemorrhage to VH was 3.5±1.2 weeks (ranging from 2 to 6 weeks). All of the eyes had massive subretinal haemorrhages (the mean subretinal haemorrhage area was 16.7±7.1 in disc size).
Patients underwent PPV 1.3±0.7 months (ranging from 0.1 to 2.5 months) after the onset of the VH. Bevacizumab was injected intravitreally into nine eyes during the operation. The eye with RD and VH after pneumatic displacement received PPV and gas tamponade, and the retina attached postoperatively. No eye developed any complication after PPV, except for cataracts. There was no recurrent VH during the postoperative follow-up. Active PCV lesions were found in five eyes, and bevacizumab was injected. One eye (patient no. 8) developed new PCV lesion with small subretinal haemorrhage at 1 year after vitrectomy. Bevacizumab was injected two times. The haemorrhage resolved and final BCVA was 20/100 (LogMAR 0.7).
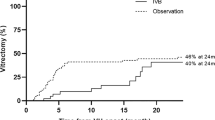
Baseline visual acuity was LogMAR 0.95±0.60 before VH (measured at the time of subretinal haemorrhage) and dropped to 2.63±0.57 before PPV. Visual acuity improved to LogMAR 1.62±0.67 at 3 months postoperatively (P<0.001) and LogMAR 1.43±0.82 at the final visit (P<0.001) (Figure 1a). All eyes showed improvement in visual acuity, except one eye that was limited by dense cataract and marked macular scarring (Figure 1b). Final BCVA ranged from HM (LogMAR 3.0) to 20/25 (LogMAR 0.1) and was not correlated with age, gender, systemic disease, subretinal haemorrhage size, duration from subretinal haemorrhage to VH, duration from VH to PPV, preoperative treatment with intravitreal bevacizumab injection, or PDT. In the six eyes with initial polyps at the subfoveal or juxtafoveal areas, one eye (16.70%) achieved final BCVA ≥20/400 (LogMAR 1.3); in the other eight eyes with initial polyps at the extrafoveal area, seven eyes (87.50%) had final BCVA ≥20/400 (Fisher’s exact test, P=0.026).
(a) Change of VA: baseline VA was LogMAR 0.95±0.60 before VH and dropped to 2.63±0.57 before vitrectomy. VA improved to LogMAR 1.62±0.67 at 3 months postoperatively and final VA was LogMAR 1.43±0.82. (P<0.001 for preoperative LogMAR and final LogMAR). (results represent mean±SD) (b) Final postoperative visual acuity plotted against preoperative visual acuity. All eyes had improved visual acuity at final visit compared with preoperative visual acuity, except for one eye with dense cataract and marked macular scarring. The color reproduction of this figure is available on the Eye journal online.
Case presentation
Patient No. 3 (Figure 2): A 55-year-old woman without any systemic disease, presented with blurred vision in the right eye for 3 days. Visual acuity was 20/100 (LogMAR 0.7), and fundus examination demonstrated massive subretinal haemorrhage. ICG showed extrafoveal polypoidal lesions. PDT with spot size 5000 μm and power 600 mW/cm2 was performed for 83 s. VH occurred 1 week after PDT, and the visual acuity dropped to 20/500 (LogMAR 1.4). Ultrasonography demonstrated hypoechoic opacities in the vitreous cavity and subretinal space. PPV was performed for non-clearing VH 2 months later. The final BCVA (4 years postoperatively) was 20/30 (LogMAR 0.18) with retinal pigment epithelial changes at the lower part of macula.
Patient No. 3. (Top left) Preoperative ICG showed extrafoveal polypoidal lesions. Visual acuity was 20/100 (LogMAR 0.7) at that time (top right) VH occurred 1 week after PDT and visual acuity dropped to 20/500 (LogMAR 1.4). Ultrasonography showed hypoechoic opacities in vitreous cavity and beneath temporal-lower retina. (Bottom left) Postoperative ICG revealed regression of polypoidal lesions. (Bottom right) Fundus picture at final visit (4 years postoperatively) demonstrated retinal pigment epithelial changes over lower part of macula. Visual acuity was 20/30 (LogMAR 0.18). The color reproduction of this figure is available on the Eye journal online.
Patient No. 10 (Figure 3): A 58-year-old man had a history of hypertension, diabetes mellitus, and valve replacement surgery with ongoing anticoagulant use. He presented with decreased vision in the left eye, and the visual acuity was 20/200 (LogMAR 1.0). Massive subretinal haemorrhage was noted. ICG revealed polypoidal lesions at the juxtafoveal area. Bevacizumab was injected intravitreally. Visual acuity declined suddenly to CF 2 days after the injection. A VH was discovered, and ultrasonography uncovered marked opacities in the vitreous and beneath the retina. PPV was performed 3 weeks after VH. The improvement of visual acuity was limited by extensive scarring, and VA decreased to HM at final visit (20 months postoperatively).
Patient No. 10. (Top left) Fundus picture at the first visit showed massive subretinal haemorrhage. (Top right) ICG revealed juxtafoveal polypoidal lesions. (Bottom left) A VH occurred 2 days after intravitreal bevacizumab injection, and visual acuity declined to CFs. Ultrasonography found marked opacities in the vitreous and beneath the retina. (Bottom right) The fundus picture at 2 months postoperatively revealed extensive submacular haemorrhage and exudate. Visual acuity at that time was 20/2000 (LogMAR 2.0). The improvement of visual acuity was limited by extensive scarring, and visual acuity decreased to HM at the final visit (20 months postoperatively).
Discussion
PCV usually presents with recurrent serous and haemorrhagic detachments of the RPE, and massive haemorrhage occurs occasionally with sudden deterioration of vision.18, 19 The cluster type of PCV lesions, which was the most common formation of the PCV lesions, was found to be related to increased frequency of massive haemorrhage with poorer visual outcomes.7, 16 Larger PCV size (lesion ≥1 disc area) is associated with severe vision-threatening complications, such as suprachoroidal haemorrhage and VH.20 In some genotype–phenotype analysing studies, the risk genotypes of LOC387715 rs10490924 were correlated with larger lesion size, greater chance of VH, and worse therapeutic response.21, 22, 23
VH associated with PCV can occur as the initial presentation or during the follow-up period.14, 15, 24, 25 In this current study, VH was the initial presentation in two eyes, whereas seven eyes were found to have spontaneous breakthrough VH from subretinal haemorrhage during the follow-up period. Ultrasonography was crucial in the setting of VH obscuring fundus detail and helped to exclude more urgent aetiologies. In a study describing the ultrasonographic features of PCV with VH, two characteristic types of ultrasonographic findings were reported that might represent the different stages of the haemorrhagic retinal elevations.15 The most important sign was the detection of hypoechoic opacities on both sides of a hyper-reflective membrane (Figure 2, top right and Figure 3, bottom left), indicating haemorrhages distributed both in the vitreous cavity and in the subretinal space. In contrast, the area under the retinal membrane would be echo free in rhegmatogenous RD. The other ultrasonographic feature of the PCV lesion depicted a dome-shaped elevation with hypoechoes inside and even a fluid level, representing haemorrhagic pigment epithelial detachment.15 Ultrasonography could also help to exclude other possible causes of VH, such as intraocular tumours or posterior vitreous detachment.
PCV-related breakthrough VH can also develop after manipulations, such as PDT,11, 12, 26 intravitreal injection of anti-VEGF agents,10 pneumatic displacement,13 or combined therapy with PDT and anti-VEGF agents.27, 28, 29 In the current study, three cases of VH developed after PDT. Four eyes received intravitreal bevacizumab injections, and one eye had pneumatic displacement before the onset of breakthrough VH. Postoperative bleeding is a known complication of PDT for PCV11, 12 that results in subretinal haemorrhage in 31% of treated eyes and VH in 6.6–12.5% of treated eyes.12, 26 The risk of massive VH after PDT was higher in PCV than in common AMD, and VH was indicative of poor visual prognosis.12 Larger laser irradiation spot size (especially >3500 μm) of PDT was considered to be a higher risk for postoperative VH.12 Three cases in the current study received PDT in eyes with pre-existing subretinal haemorrhages and developed VH 1–6 weeks after the therapy. The laser spot sizes used in this study were 1500, 3500, and 5000 μm, respectively. The mechanism of haemorrhage might be related to the direct effects of laser exposure or to altered choroidal blood flow.12 PDT would cause occlusion of both the abnormal and physiologic choroidal vessels within the treated area, resulting in increased blood flow in fragile vasculatures and leading to massive haemorrhage complications.12
Although it is controversial whether combination of intravitreal injection of anti-VEGF agents with PDT could decrease the risk of haemorrhage complication after PDT,26, 27, 28, 29 intravitreal injection of anti-VEGF agents alone could also induce haemorrhage. Cho et al10 retrospectively reviewed 54 patients with PCV who had received intravitreal ranibizumab injection. They found postoperative subretinal haemorrhages in five (8.9%) eyes and VH in one (1.8%) eye. All subretinal haemorrhages developed within 1 week after injection. Those with large PCV lesions (size ≥15 mm2) carried a higher risk for bleeding. Visual acuity decreased significantly in the eye with postoperative VH. The mechanism of haemorrhagic complications after the intravitreal ranibizumab injection could be an RPE tear,30 resulting from the contraction of choroidal vessels or changes in choroidal blood flow following ranibizumab injection.10 In the current study, VH occurred 1 to 4 days following the intravitreal bevacizumab injection in the eyes with pre-existing subretinal haemorrhage, suggesting that intravitreal injection might be a trigger factor for breakthrough VH. It could be postulated that intravitreal injection might cause intraocular pressure fluctuations within short durations of time and stretch of choroid and sclera in the eyes with a massive subretinal haemorrhage that might lead to breakthrough VH.
Wu and co-workers13 retrospectively evaluated 120 eyes that had undergone intravitreal tissue plasminogen activators and pneumatic displacements for submacular haemorrhages. Eighteen eyes (15%) reported complications with breakthrough VH, and two cases (1.67%) suffered from RD. They found a large area of submacular haemorrhage (≥10 disc areas) and PCV as the underlying aetiology were associated with a higher risk of VH after the procedure, and the occurrence of VH did not affect the visual prognosis of submacular haemorrhage. There was only one eye that received pneumatic displacement in our study, and RD accompanied with VH was found 10 days after the procedure. Although PPV and gas tamponade had anatomically successful results, the final VA was restricted by macular scarring and recurrent subretinal haemorrhage.
In the current study, VH occurred 2–6 weeks (3.5 weeks on average) after subretinal haemorrhage, which concurs with the observations of previous studies.14, 31 Further, all of the eyes in this study had massive subretinal haemorrhages (average haemorrhage area: 16.7 disc size), which suggests that eyes with massive subretinal haemorrhages were at risk of breakthrough VH. Several common characteristics of breakthrough VH recognized in earlier reports, as well as in this study, helped to clarify the mechanism of spontaneous breakthrough VH. VH usually developed several weeks following subretinal haemorrhage, and turbid, thick, organized haemorrhages were seen even in newly developed VH.14 No breaks could be identified in such cases. Lincoff et al32 demonstrated in a rabbit model that subretinal blood could cause necrosis of the overlying retina, except for the internal limiting membrane and that fragments of erythrocytes could migrate across the damaged retina into the vitreous cavity. Manipulations such as intravitreal injection of anti-VEGF agents, PDT, and pneumatic displacement may trigger the migration of the subretinal blood that clouds the vitreous.10, 11, 12, 13, 26
Few studies have reported the visual outcomes of PPV for PCV with breakthrough VH (Table 2).10, 12, 14, 15 Jalali et al15 reported 10 eyes receiving PPV combined with individualized procedures for VH associated with PCV. The mean follow-up duration was 19.5 months. PPV was performed 3.1 months after the onset of VH. Preoperative visual acuity measured light perception (LP) only, and the final visual acuity ranged from LP to 20/25 (LogMAR 0.1), including six eyes (60%) with BCVA ≥20/400 (LogMAR 1.3). Surgically related complications consisted of rhegmatogenous RDs in three eyes. Jung et al14 investigated 12 eyes that underwent PPV for PCV with VH. The mean follow-up duration was 12.7 months. The preoperative BCVA was LP to 20/80 (LogMAR 0.6), and the final BCVA ranged from CFs to 20/25 (LogMAR 0.1), including BCVA ≥20/400 (LogMAR 1.3) in seven eyes (54%). Surgical complications included one choroidal detachment. In our study, visual outcomes varied from HM to 20/25 (LogMAR 0.1). The percentage of eyes with final BCVA ≥20/400 (LogMAR 1.3) was 47.1%, slightly lower than the results (54–60%) of previous studies.14, 15 A longer follow-up duration with a higher rate of recurrence and cataract formation might account for this finding. PPV could facilitate earlier diagnosis and provide ambulatory vision even for those with macular lesions and central scotoma. With careful management, none of our cases experienced any of the possible surgical complications, such as retinal breaks, RDs, or choroidal detachments.
During postoperative follow-up, recurrent VH or new PCV lesions had been reported.14, 15 Jung et al14 reported recurrent VH after PPV in four cases, which absorbed spontaneously in three eyes. Two eyes developed new PCV lesions within 1 year and was managed by laser photocoagulation in Jalali et al’s study.15
In the present study, there was no recurrent VH during the postoperative follow-up. New PCV lesion with small subretinal haemorrhage occurred in one eye at 1 year after PPV and was treated successfully with intravitreal bevacizumab injection. Long-term follow-up is necessary in cases with PCV-related breakthrough VH to recognize and manage the recurrent lesions earlier.
Several reports studying vitrectomy for AMD-related VH demonstrated poor functional outcomes, possibly due to severe inflammatory reactions, extensions of pre-existing subretinal haemorrhages, and surgical complications.33, 34, 35 However, our study and other studies14, 15 reported more favourable visual outcomes of vitrectomy for PCV-related VH. Visual recovery was limited mainly by macular pathology, such as macular scarring, atrophy, or RPE changes. Subretinal blood can act as a barrier to block metabolic exchange, causing degenerative changes of the RPE and outer retina.36 In this study, none of the following were shown to be related to visual outcomes: age, gender, subretinal haemorrhage size, duration from subretinal haemorrhage to VH, duration from VH to PPV, preoperative treatment with intravitreal bevacizumab injection, or PDT. Cases with systemic disease (DM or HTN) showed a borderline relationship (Fisher’s exact test, P=0.05) with poorer visual outcomes. Eyes with initial polyps at the extrafoveal area had better visual outcomes than those with polyps at the subfoveal or juxtafoveal areas. Foveal microstructures were more likely to be destroyed, as the polyps close to the fovea restricted visual recovery. In a recent study comparing the long-term results of PDT with or without intravitreal bevacizumab injection for PCV, the polyp location was more significantly associated with the final visual improvement rather than treatment modality itself.37
The limitations of this study include its retrospective study design, small case numbers, and potential confounding factors. Nevertheless, our study has the largest sample size and the longest follow-up duration compared with other studies. These results still provide valuable information for clinical practice.
In summary, the visual prognosis in eyes with PCV-related breakthrough VH is variable after vitrectomy. Massive subretinal haemorrhage is at risk for breakthrough VH. Some treatment modalities, such as intravitreal injection, pneumatic displacement, and PDT, may trigger the development of breakthrough VH. Visual recovery after PPV may be limited by macular pathology, and initial polyps at the subfoveal or juxtafoveal areas may be linked to a poorer functional prognosis. Further prospective study is required for better understanding of the surgical outcomes of PCV-related breakthrough VH.

References
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B . Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990; 10 (1): 1–8.
Kleiner RC, Brucker AJ, Johnston RL . The posterior uveal bleeding syndrome. Retina 1990; 10 (1): 9–17.
Stern RM, Zakov ZN, Zegarra H, Gutman FA . Multiple recurrent serosanguineous retinal pigment epithelial detachments in black women. Am J Ophthalmol 1985; 100 (4): 560–569.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA . Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995; 15 (2): 100–110.
Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 2003; 121 (10): 1392–1396.
Kwok AK, Lai TY, Chan CW, Neoh EL, Lam DS . Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol 2002; 86 (8): 892–897.
Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 2002; 133 (5): 639–648.
Hou J, Tao Y, Li XX, Zhao MW . Clinical characteristics of polypoidal choroidal vasculopathy in Chinese patients. Graefes Arch Clin Exp Ophthalmol 2011; 249 (7): 975–979.
Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology 2004; 111 (8): 1576–1584.
Cho HJ, Lee DW, Cho SW, Kim CG, Kim JW . Hemorrhagic complications after intravitreal ranibizumab injection for polypoidal choroidal vasculopathy. Can J Ophthalmol 2012; 47 (2): 170–175.
Ojima Y, Tsujikawa A, Otani A, Hirami Y, Aikawa H, Yoshimura N . Recurrent bleeding after photodynamic therapy in polypoidal choroidal vasculopathy. Am J Ophthalmol 2006; 141 (5): 958–960.
Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, Mandai M et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina 2007; 27 (3): 335–341.
Wu TT, Kung YH, Hong MC . Vitreous hemorrhage complicating intravitreal tissue plasminogen activator and pneumatic displacement of submacular hemorrhage. Retina 2011; 31 (10): 2071–2077.
Jung JH, Lee JK, Lee JE, Oum BS . Results of vitrectomy for breakthrough vitreous hemorrhage associated with age-related macular degeneration and polypoidal choroidal vasculopathy. Retina 2010; 30 (6): 865–873.
Jalali S, Parra SL, Majji AB, Hussain N, Shah VA . Ultrasonographic characteristics and treatment outcomes of surgery for vitreous hemorrhage in idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol 2006; 142 (4): 608–619.
Cackett P, Wong D, Yeo I . A classification system for polypoidal choroidal vasculopathy. Retina 2009; 29 (2): 187–191.
Holladay JT . Visual acuity measurements. J Cataract Refract Surg 2004; 30 (2): 287–290.
Ahuja RM, Stanga PE, Vingerling JR, Reck AC, Bird AC . Polypoidal choroidal vasculopathy in exudative and haemorrhagic pigment epithelial detachments. Br J Ophthalmol 2000; 84 (5): 479–484.
Tsujikawa A, Sasahara M, Otani A, Gotoh N, Kameda T, Iwama D et al. Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am J Ophthalmol 2007; 143 (1): 102–111.
Tsujikawa A, Ojima Y, Yamashiro K, Nakata I, Ooto S, Tamura H et al. Association of lesion size and visual prognosis to polypoidal choroidal vasculopathy. Am J Ophthalmol 2011; 151 (6): 961–972 and 961.
Park DH, Kim IT . Association of ARMS2/HTRA1 variants with polypoidal choroidal vasculopathy phenotype in a Korean population. Jpn J Ophthalmol 2012; 56 (1): 60–67.
Chen H, Liu K, Chen LJ, Hou P, Chen W, Pang CP . Genetic associations in polypoidal choroidal vasculopathy: a systematic review and meta-analysis. Mol Vis 2012; 18: 816–829.
Sakurada Y, Kubota T, Mabuchi F, Imasawa M, Tanabe N, Iijima H . Association of LOC387715 A69S with vitreous hemorrhage in polypoidal choroidal vasculopathy. Am J Ophthalmol 2008; 145 (6): 1058–1062.
Uyama M, Matsubara T, Fukushima I, Matsunaga H, Iwashita K, Nagai Y et al. Idiopathic polypoidal choroidal vasculopathy in Japanese patients. Arch Ophthalmol 1999; 117 (8): 1035–1042.
Lip PL, Hope-Ross MW, Gibson JM . Idiopathic polypoidal choroidal vasculopathy: a disease with diverse clinical spectrum and systemic associations. Eye (Lond) 2000; 14 (Part 5): 695–700.
Nakata I, Tsujikawa A, Yamashiro K, Otani A, Ooto S, Akagi-Kurashige Y et al. Two-year outcome of photodynamic therapy combined with intravitreal injection of bevacizumab and triamcinolone acetonide for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 2013; 251 (4): 1073–1080.
Tomita K, Tsujikawa A, Yamashiro K, Ooto S, Tamura H, Otani A et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy combined with intravitreal injections of ranibizumab. Am J Ophthalmol 2012; 153 (1): 68–80, e61.
Sato T, Kishi S, Matsumoto H, Mukai R . Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for polypoidal choroidal vasculopathy. Am J Ophthalmol 2010; 149 (6): 947–954, e941.
Gomi F, Sawa M, Wakabayashi T, Sasamoto Y, Suzuki M, Tsujikawa M . Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2010; 150 (1): 48–54, e41.
Bakri SJ, Kitzmann AS . Retinal pigment epithelial tear after intravitreal ranibizumab. Am J Ophthalmol 2007; 143 (3): 505–507.
Googe JM, Hirose T, Apple DJ, Melgen S . Vitreous hemorrhage secondary to age-related macular degeneration. Surv Ophthalmol 1987; 32 (2): 123–130.
Lincoff H, Madjarov B, Lincoff N, Movshovich A, Saxena S, Coleman DJ et al. Pathogenesis of the vitreous cloud emanating from subretinal hemorrhage. Arch Ophthalmol 2003; 121 (1): 91–96.
Orth DH, Flood TP . Management of breakthrough vitreous hemorrhage from presumed extramacular subretinal neovascularization. Retina 1982; 2 (2): 89–93.
Azzolini C, Menchini U, Pece A, Camesasca F, Giuliani V . Age-related macular degeneration and vitreous hemorrhage. Eur J Ophthalmol 1991; 1 (3): 142–147.
Roufail E, Polkinghorne PJ . Combined cataract surgery and vitrectomy for vitreous haemorrhage secondary to age-related macular degeneration. Clin Exp Ophthalmol 2008; 36 (1): 36–38.
Reynders S, Lafaut BA, Aisenbrey S, Broecke CV, Lucke K, Walter P et al. Clinicopathologic correlation in hemorrhagic age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2002; 240 (4): 279–285.
Lee YA, Yang CH, Yang CM, Ho TC, Lin CP, Huang JS et al. Photodynamic therapy with or without intravitreal bevacizumab for polypoidal choroidal vasculopathy: two years of follow-up. Am J Ophthalmol 2012; 154 (5): 872–880, e2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lin, HC., Yang, CH. & Yang, CM. Visual outcomes of vitrectomy for polypoidal choroidal vasculopathy-related breakthrough vitreous haemorrhage. Eye 28, 797–807 (2014). https://doi.org/10.1038/eye.2014.124
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.124
This article is cited by
-
Optical coherence tomography-based misdiagnosis and morphological distinction in pachychoroid neovasculopathy vs. polypoidal choroidal vasculopathy
Eye (2023)
-
Surgical outcomes of vitrectomy for breakthrough vitreous hemorrhage in eyes with exudative age-related macular degeneration
International Ophthalmology (2021)
-
Long-term Clinical Course after Vitrectomy for Breakthrough Vitreous Hemorrhage Secondary to Neovascular Age-related Macular Degeneration and Polypoidal Choroidal Vasculopathy
Scientific Reports (2020)
-
Vitreous haemorrhage in massive hemorrhagic polypoidal choroidal vasculopathy: clinical characteristics and surgical outcomes
International Journal of Retina and Vitreous (2015)