Abstract
Aim
To evaluate the efficacy of intravitreal bevacizumab in the treatment of retinal vasoproliferative tumours (VPT).
Materials and Methods
Six eyes of 6 patients with VPT who received intravitreal bevacizumab were retrospectively reviewed. All patients received between one and three injections of intravitreal bevacizumab depending upon response to treatment. Best-corrected visual acuity (BCVA), tumour size, and presence of co-pathology or sequelae were noted pre- and postoperatively and then analysed. Subsequent retreatments were performed in patients with recurrent or persistent VPT according to the ophthalmologist’s discretion. Retreatments included photodynamic therapy with verteporfin, ruthenium-106 plaque brachytherapy, or endoresection of tumour.
Results
The mean follow-up duration was 33.3 months (range 10–66 months). At baseline, the mean logMAR BCVA was 1.45 (Snellen equivalent of 6/165); range 0.10–1.90 (6/8—CF). Following bevacizumab treatment the mean logMAR BCVA was 0.98 (Snellen equivalent of 6/57); range 0.5–1.9 (Snellen equivalent of 6/19 to CF). Therefore, there was no statistically significant change in visual acuity. The mean tumour thickness reduced from 2.4 to 2.1 mm following treatment with bevacizumab. However, this did not reach the statistical significance of P<0.05. Despite the visual improvement following bevacizumab therapy, five out of six patients had recurrence of tumour activity during the follow-up period and required further intervention in order to achieve sustained regression.
Conclusions
Intravitreal bevacizumab appeared to result in temporary reduction of tumour thickness in 3 out of 6 VPT patients. However, neither the reduction in tumour thickness nor the change in visual acuity were statistically significant and intravitreal bevacizumab monotherapy had limited effectiveness in causing long-term regression of the lesions. Additional therapy was indicated in five out of six patients to establish long-term regression. The efficacy of bevacizumab as an adjunct is as yet undetermined and further studies are needed. Presently, we recommend other treatment modalities in the long-term management of VPTs.
Similar content being viewed by others
Introduction
Vasoproliferative tumours of the retina (VPTs) are benign lesions of unknown origin and have been treated with different modalities with varying success. They are characterised by a pink to yellow appearance on funduscopy and are often accompanied by exudative and haemorrhagic changes of the retina. VPTs are highly vascularised tumours, often secondary to other pathology, and histologically represent reactive gliovascular proliferations.1, 2 This suggests that VEGF is likely to be involved in the proliferative pathway of VPT formation and, as such, may be susceptible to treatment with anti-VEGF treatment.
VEGF is an appropriate treatment target for such conditions because of its propensity to cause angiogenesis and vascular permeability. The humanised monoclonal antibody bevacizumab (Avastin; Genentech/Roche, San Francisco, CA, USA) is one of several anti-VEGF treatments currently being used for the treatment of choroidal neovascularisation in age-related macular degeneration.3 There have been reports of success with bevacizumab in the treatment of both diabetic4 and radiation retinopathy.5 Avery et al4 reported complete (or at least partial) reduction in leakage of neovascularisation in patients with proliferative diabetic retinopathy within 1 week after intravitreal injection of bevacizumab.
Our group have previously reported a case of resolution of VPT with a single intravitreal injection of bevacizumab.6 We were therefore keen to further explore whether bevacizumab was a useful treatment in patients with VPT and whether long-term regression could be induced.
Materials and methods
This was a retrospective study of patients who had intravitreal bevacizumab for the treatment of VPT from September 2006 to February 2011. The inclusion criteria of the study included: age of ≥18 years and treatment with intravitreal bevacizumab. There were no exclusion criteria.
All participants underwent ocular examination including best-corrected visual acuity (BCVA) testing, intraocular pressure assessment, dilated fundus examination, and ultrasound B-scan. BCVA was measured using an ETDRS logMAR chart at 4 m or with a standard Snellen chart at 6 m converted to logMAR visual acuity for analysis. The decision to treat was based upon tumour activity. This was defined as: reduced BCVA, increased tumour size on USS, and the presence of exudative RD with or without macular exudates.
Intravitreal bevacizumab injection was performed under topical anaesthesia as an outpatient procedure. Intravitreal injection of 1.25 mg bevacizumab (Avastin) in 0.05 ml was carried out using an aseptic technique at 6-weekly intervals.
Additional treatments were performed in eyes with persistent or recurrent active VPT identified at follow-up. Follow-up assessment included ocular examination including BCVA testing, intraocular pressure assessment, dilated fundus examination, and ultrasound B-scan. Ocular coherence topography and fundus fluoroscein angiography were performed at the ophthalmologists discretion. The retreatment modality was performed according to the ophthalmologist’s discretion that included PDT with verteporfin, ruthenium-106 plaque brachytherapy, or endoresection of tumour. Success was considered to be inactivation of the tumour and was defined as stabilisation or improvement of BCVA with stabilisation of tumour size on USS and resolution of exudative retinal detachment and macular oedema.
Nonparametric analyses for continuous variables were compared using the Wilcoxon matched-pairs test (Pratt’s method). A P-value of <0.05 was considered as statistically significant. Patients 2 and 3, who presented with an ERM, were not included in statistical testing for BCVA so that they could not skew the results.
Results
Six eyes of 6 patients were recruited, and the details are listed in Table 1: demographics and outcomes. The mean age of the patients was 41.5 years (range 19–60 years). The mean follow-up duration was 33.33 months (range 10–66 months).
At baseline, the mean logMAR BCVA was 1.45 (Snellen equivalent of 6/165); range 0.10–1.90 (6/8—CF). Following bevacizumab treatment, the mean logMAR BCVA was 0.98 (Snellen equivalent of 6/57); range 0.5–1.9 (Snellen equivalent of 6/19 to CF). The P-value was 0.5, and therefore there was no statistically significant change in visual acuity.
The mean tumour thickness reduced from 2.4to 2.1 mm; (range +0.2 to −1.2 mm). A Wilcoxon matched-pairs test was performed and the P-value was 0.38. Therefore, this reduction in tumour thickness was not considered to be statistically significant.
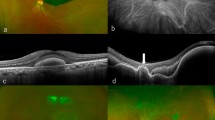
Following bevacizumab therapy, five out of six patients had recurrence of tumour activity during the follow-up period and required further intervention in order to achieve sustained regression; with only the patient with the shortest follow-up period requiring no further intervention as yet. Of the four patients who presented with exudative retinal detachment, three required vitreoretinal surgery for resolution. Two had endoresection of tumour and one had PPV and cryotherapy to induce regression of tumour activity and resolution of exudative RD. Patient 3 had resolution of macular oedema follwing photodynamic therapy.
Discussion
VPTs are highly vascularised tumours and, as such, may be susceptible to anti-VEGF treatments. It is likely that VEGF is involved in the aetiology of vasoproliferative tumours. Their natural history involves neovascularisation, leakage of exudates, and tractional retinal detachment. Reduction in visual acuity is largely through the effects of the tumour upon the macula, such as macular oedema or epiretinal membrane formation.7 Anti-VEGF should decrease leakage and improve macular oedema as well as inhibit neovascularisation and induce regression of new vessels.4
Within our department, one case of VPT has been treated with bevacizumab and underwent complete regression of the tumour after just one intravitreal injection. This case has already been reported by Kenawy et al.6 Saito et al8 have recently reported two patients whose VPTs regressed following a single dose of bevacizumab. These patients had small tumours measuring less than 2 disc diameters.
Given such success, we were keen to explore whether this treatment works by studying a larger cohort with longer follow-up in an effort to determine whether these effects are reproducible and whether sustained tumour regression may be induced.
Our patients had an average follow-up period of 33.3 months (range 10–66 months). Patients were discharged from follow-up and referred back to their referring clinician when the tumour had regressed and any sequelae had been managed.
BCVA improved from an average of 1.45 logMAR at baseline to an average of 0.98 logMAR postoperatively. This was not found to be statistically significant. Similarly, the tumour thickness reduced from an average of 2.4 mm at baseline to 2.1 mm following intravitreal bevacizumab. This was not found to be statistically significant.
However, it is important to note that VPTs can only be defined and measured with difficulty using ultrasound echography. This is because VPTs usually have a high internal acoustic reflectivity and it may be impossible to define the exact tumour margins on ultrasound examination. Consequently, ultrasound measurements are not very reliable. We, therefore, think that the assessment of the clinical activity (tumour margins on indirect ophthalmoscopy and wide-angle photography, clinical assessment of associated exudative retinal changes, and changes of macular oedema on OCT) are the best way of assessing tumour activity and the need for retreatment.
Based on the clinical assessment, five of six patients needed further intervention in order to achieve sustained regression. Only one patient developed ERM following intravitreal bevacizumab treatment and no other significant complications or long-term systemic side effects were detected.
In his recent article, Rennie9 highlighted how the rarity of VPT has resulted in a lack of an evidence-based consensus agreement on the best treatment. He suggested that if the visual acuity is effected or threatened or if a significant amount of exudate or traction is associated with a lesion intervention is required. He suggested that small peripheral tumours that are asymptomatic may be observed.
Shields et al7 effectively treated active VPTs using triple freeze thaw transconjunctival cryotherapy, but the side effects include scleral thinning with discolouration and vitreous haemorrhage. Recent studies have demonstrated that VPT can be treated with either ruthenium-106 (see Anastassiou et al10) or iodine-125 (see Cohen et al11) plaque brachytherapy.
Anastassiou et al10 have reported their results with 35 consecutive patients with VPT treated with ruthenium-106 plaque radiotherapy. They achieved tumour regression and resolution of the exudation in 31 patients (89%). Visual acuity deteriorated in 15 of 35 patients, with 5 of these having severe visual loss after a median follow-up of 24 months. They declared the main cause of visual loss as epiretinal membrane formation, with 10/35 (28.6%) patients having ERM after ruthenium-106 therapy.
Cohen et al11 reported tumour regression in 29/30 (97%) of patients treated with iodine-125. They found that the vision was stable or improved in 22/30 (73%) of patients.
Heimann et al2 found regression of tumour and exudates in all treated eyes in their study of 22 eyes with VPT but noted that visual acuity was worse by >2 Snellen lines in 5 of these 22 eyes. Of the treated 19 eyes, 18 were managed successfully with plaque radiotherapy, cryotherapy, or a combination of the two. They concluded that both plaque radiotherapy and cryotherapy were useful in the management of VPT.
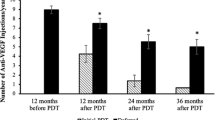
Blasi et al12 reported resolution of macular oedema and reduction in tumour thickness in three patients treated with photodynamic therapy and Verteporfin. However, the follow-up period was limited to 1 year and the authors concluded that further studies with larger sample sizes were needed.
Several case reports have recently appeared in the literature. Bertelli and Pernter13 reported a single case of complete obliteration of a VPT and resolution of oedema following treatment with indocyanine green-mediated photothrombosis. Japiassu et al14 recently reported a regression of VPT following the use of systemic infliximab to treat a patient suffering from mixed connective tissue disease. It is unknown whether such treatment is specific to this patient subset or whether it would have any effect in patients with primary tumours and other secondary tumours.
Although visual acuity is arguably the most significant outcome measure to the patient, unfortunately visual improvement does not always follow reduction of tumour size or resolution of exudates. In our study the visual acuity remained stable or improved in four of the six patients. The other two patients each had epiretinal membranes, one of which was detected at presentation and the other developed following treatment with intravitreal bevacizumab. Their BCVA data were excluded from the analysis.
The mean post-treatment BCVA was logMAR 0.98 (Snellen equivalent of 6/57) despite tumour regression and resolution of exudates in 5 out of the 6 patients. This may, in part, be logically explained by the presence of macular pathology and also by the fact that two eyes were amblyopic and the aim of treatment was to stabilise the tumour rather than improve visual acuity.
VPTs may have a variety of co-pathology, some of which may play a role in the aetiology and some may be sequelae of the tumour itself or the treatment. In our study an ERM was detected following treatment with bevacizumab in one patient and two patients had ERM noted before treatment. This made it difficult to determine whether ERMs are sequelae of the treatment or secondary to the continued activation of the vasoproliferative tumour itself or, indeed, whether the treatment reduced oedema enabling the detection of a preexisting ERM. We suggest that the presence of ERM as the only cause for decreased visual acuity should be considered an exclusion criteria for treatment with bevacizumab.
In our study, only one patient (who had the shortest follow-up) appeared to be in tumour regression/quiescence following treatment. Only three of the six patients showed any reduction in tumour thickness following intravitreal bevacizumab and, of those, only one appeared to be in regression, with five of six patients requiring further intervention to induce sustained tumour regression. Similarly, Saito et al8 described two of nine patients whose vasoproliferative tumours regressed following a single dose of bevacizumab. These patients had small tumours measuring less than 2 disc diameters. The remaining seven patients required additional treatments in order to induce tumour regression and resolution of exudative retinal detachments.
This suggests that either there is only a short-term efficacy of bevacizumab or that there is a persistent stimulus for tumour activity that lasts after the effects of bevacizumab subside.
Conclusion
We conclude that although we found no clear evidence of harmful effects of intravitreal bevacizumab monotherapy, there were no clinically significant beneficial effects nor does it induce any lasting regression of vasoproliferative tumours. The presence of ERM as the only cause for decreased visual acuity should be considered an exclusion criterion for treatment with bevacizumab. The use of bevacizumab as an adjunctive therapy is undetermined and further studies are needed. Therefore, we recommend other treatment modalities and do not recommend intravitreal bevacizumab monotherapy in the long-term treatment of vasoproliferative tumours.

References
Damato B . Vasoproliferative retinal tumour. Br J Ophthalmol 2006; 90: 399–400.
Heimann H, Bornfeld N, Vij O, Coupland SE, Bechrakis NE, Kellner U et al. Vasoproliferative tumours of the retina. Br J Ophthalmol 2000; 84: 1162–1169.
Shah AR, Del Priore LV . Duration of action of intravitreal ranibizumab and bevacizumab in exudative AMD eyes based on macular volume measurements. Br J Ophthalmol 2009; 93: 1027–1032.
Avery R, Pearlman J, Pieramici D, Rabena M, Castellarin A, Nasir M et al. Intravitreal Bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006; 113: 1695–1705.
Finger P T . Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Physiol 2008; 70: 974–977.
Kenawy N, Groenwald C, Damato B . Treatment of a vasoproliferative tumour with intravitreal bevacizumab (Avastin). Eye 2007; 21: 893–894.
Sheilds CL, Sheilds JA, Barrett J, De Potter P . Vasoproliferative tumours of the ocular fundus. Classification and clinical manifestations in 103 patients. Arch Ophthalmol 1995; 113: 615–623.
Saito W, Kase S, Fujiya A, Dong Z, Noda K, Ishida S . Expression of vascular endothelial growth factor and intravitreal anti-VEGF therapy with bevacizumab in vasoproliferative retinal tumors. Retina 2013; 33: 1959–1967.
Rennie IG . Retinal vasoproliferative tumours. Eye 2010; 24: 468–471.
Anastassiou G, Bornfeld N, Schueler AO, Schilling H, Weber S, Fleuhs D et al. Ruthenium-106 plaque brachytherapy for symptomatic vasoproliferative tumours of the retina. Br J Ophthalmol 2006; 90: 447–450.
Cohen VML, Sheilds CL, Demirci H, Sheilds JA . Iodine-125 plaque radiotherapy for vasoproliferative tumors of the retina in 30 eyes. Arch Ophthalmol 2008; 126: 1245–1251.
Blasi MA, Scupola A, Tiberti AC, Sasso P, Balestrazzi E . Photodynamic therapy for vasoproliferative retinal tumors. Retina 2006; 26: 404–409.
Bertelli E, Pernter H . Vasoproliferative retinal tumor treated with indocyanine green-mediated photothrombosis. Retin Cases Brief Rep 2009; 3: 266–271.
Japiassu RM, Brasil OFM, Cunha AL, de Souza EC . Regression of vasoproliferative tumor with systemic infliximab. Ophthalmic Surg Lasers Imaging 2008; 39: 348–349.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rogers, C., Damato, B., Kumar, I. et al. Intravitreal bevacizumab in the treatment of vasoproliferative retinal tumours. Eye 28, 968–973 (2014). https://doi.org/10.1038/eye.2014.113
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2014.113