Abstract
Purpose
To investigate serial changes of the Ahmed glaucoma valve (AGV) implant tube in the anterior chamber by anterior segment optical coherence tomography (AS-OCT).
Methods
Patients who had received AGV implantation without complications (n=48) were included in this study. Each patient received follow-up examinations including AS-OCT at days 1 and 2, week 1, and months 1, 3, 6, and 12. Tube parameters were defined to measure its length and position. The intracameral length of the tube was from the tip of the bevel-edged tube to the sclerolimbal junction. The distance between the extremity of the tube and the anterior iris surface (T–I distance), and the angle between the tube and the posterior endothelial surface of the cornea (T–C angle) were defined. Factors that were related to tube parameters were analysed by multiple regression analysis.
Results
The mean change in tube length was −0.20±0.17 mm, indicating that the tube length shortened from the initial inserted length. The mean T–I distance change was 0.11±0.07 mm and the mean T–C angle change was −6.7±5.6°. Uveitic glaucoma and glaucoma following penetrating keratoplasty showed the most changes in tube parameters. By multiple regression analysis, diagnosis of glaucoma including uveitic glaucoma (P=0.049) and glaucoma following penetrating keratoplasty (P=0.008) were related to the change of intracameral tube length.
Conclusions
These results suggest that the length and position of the AGV tube changes after surgery. The change was prominent in uveitic glaucoma and glaucoma following penetrating keratoplasty.
Similar content being viewed by others
Introduction
Ahmed glaucoma valve (AGV) implantation surgery creates an alternative pathway for aqueous outflow from within the eye to a silicone base plate covered by Tenon’s tissue and the conjunctiva. The AGV consists of a flexible silicone base plate with a sheet-like silicone valve, equipped with a silicone tube of 0.64 mm in diameter. During insertion, the AGV is fixed to the sclera with a nonabsorbable suture, forming a filtering bleb far away from the limbal region. The silicone tube is the only pathway from the anterior chamber to the base plate and the surrounding filtering bleb. Achieving the desired tube length and position may be quite difficult. If the tube is too close to the corneal endothelial surface, it can aggravate endothelial cell loss.1, 2 Alternatively, attempts to position the tube closer to the iris can lead to iris bleeding and tube obstruction with iris. Alterations in tube positioning can occur as an immediate postoperative complication that requires repositioning. Retraction of the tube has been reported as a late complication.3, 4
There were some previous works to identify the tube position and patency of drainage devices by anterior segment optical coherence tomography (AS-OCT).1, 5, 6 However, serial observation of the change of the tube length and position has not been performed. In this study, we traced the tube length and position in the anterior chamber by serial visualization of the AGV implant by AS-OCT. Using this method, we expected to observe changes in tube length and position, and sought to identify related factors.
Materials and methods
Patients scheduled for AGV implantation in the superotemporal quadrant at the Seoul St Mary’s Hospital, between November 2009 and March 2010, were considered for this prospective observational study. All patients displayed refractory glaucoma that did not respond to maximal medical treatment and/or previous trabeculectomy procedures. Only one eye per patient was enrolled.
Written informed consent was obtained from all patients. The study was approved by the Institutional Review Board of the Seoul St Mary’s Hospital, College of Medicine, the Catholic University of Korea, and was performed in accordance with The Declaration of Helsinki.
Postoperative follow-up examinations were performed on the first and second postoperative days, at week 1, and during months 1, 3, 6, and 12. Slit-lamp anterior segment examination, intraocular pressure (IOP) examination using Goldmann applantation tonometry, funduscopy, and AS-OCT were performed at each visit. Patients who did not attend each follow-up examination in the first 12 months after surgery were excluded. In addition, patients who developed postoperative complications that required additional surgical intervention were excluded.
The primary outcome measurement was the tube parameters measured from the AS-OCT images. The change of tube parameters at each visit was analysed and compared with those from the first postoperative day.
Surgical techniques
All operations were performed by one surgeon (CKP) using Model FP7 (New World Medical Inc., Rancho Cucamonga, CA, USA) with anterior chamber tube placement through a standard technique described elsewhere.7 Briefly, a fornix-based conjunctival flap was fashioned with relaxing incisions. The plate was inserted under the conjunctiva and Tenon’s capsule in the superior temporal quadrant, and secured to the sclera with nonabsorbable sutures at a distance of 8 mm from the limbus. The tube was trimmed to the correct length with a bevelled edge, typically so it extended ∼2–3 mm in the anterior chamber. Next, a paracentesis was created with a 23-gauge needle from 1 to 2 mm posterior to the limbus. Viscoelastic was injected into the anterior chamber through the same needle and the tube inserted into the anterior chamber. The tube was then loosely secured to the sclera with a 10-0 nylon suture. The extraocular portion of the tube was covered with a 4 × 4-mm-sized donor sclera that was sutured with 10-0 nylon. The conjunctiva was then closed with 8-0 vicryl sutures. Topical antibiotics and steroids were used following surgery.
Visante OCT
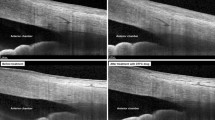
The study eye was imaged with the commercially available Visante OCT (Visante OCT Carl Zeiss Meditec, Dublin, CA, USA). The patient was asked to look at a fixation light and the upper eyelid was gently retracted by the examiner. The drainage tube was imaged using the anterior segment scan protocol by a single examiner after alignment of the scan line parallel to the axis of the drainage tube. With the cross-sectional images, measurements of the tube parameters were performed with calipers and angle tool of the device’s software by two observers (H-YLP and KIJ) in a masked fashion. Mean value of two observers was used as the measurement. The parameters of the tube were defined to measure the tube length and position. The intracameral length of the tube was from the tip of the bevel-edged to the sclerolimbal junction (Figure 1a, bottom). The distance between the extremity of the tube and the anterior iris surface (T–I distance), and the angle between the tube and the posterior endothelial surface of the cornea (T–C angle) were defined to measure the tube position (Figure 1a, top). All measurements were repeated with the cross-sectional images at days 1, 2, week 1, and months 1, 3, 6, and 12 after AGV implantation (Figures 1b and c).
Serial visualization of the anterior chamber after AGV implantation with AS-OCT. (a) Intracameral tube length, tube to iris distance (T–I distance), and angle between the tube and corneal endothelial surface (T–C angle) were measured from the cross-sectional images (postoperative day 1). Intracameral tube length was measured from the sclerolimbal junction (white arrow). (b, c) Serial images were obtained at each visit and the parameters of the tube were measured each time (b, postoperative month 1 and c, postoperative month 3).
Statistical analyses
Statistical analyses were performed using the SPSS software (ver. 12.0; SPSS, Chicago, IL, USA). Interobserver agreement of the parameter measurements were quantified by calculating the averaged measured interclass correlation coefficients (ICC).
Beseline characteristics between groups were compared by Kruskall–Wallis test or Student t-test. The parameters of the tube were compared between observation periods by Student t-test. Multiple regression analysis was used to analyse the association between the changes in tube parameters with various ocular variables. As lens status, previous trabeculectomy, and ocular diagnosis are nominal in scale, it was investigated as an independent factor using a regression model, and dummy variables were performed with the phakic lens status, no previous trabeculectomy, and primary open-angle glaucoma subgroup as a standard. A P-value ≤0.05 was considered to indicate statistical significance.
Results
Forty eight eyes of 48 patients were included in the study. Mean age of the patients was 51.54±23.46 years (range, 16–89 years). Nine eyes (18.8%) had primary open-angle glaucoma, 10 eyes (20.8%) had uveitic glaucoma, 11 eyes (22.8%) had neovascular glaucoma, nine eyes (18.8%) had glaucoma after penetrating keratoplasty, and nine eyes (18.8%) had pseudophakic glaucoma. The mean preoperative IOP was 37.1±10.7 mm Hg. Mean age (P=0.527), preoperative IOP (P=0.438), number of preoperative glaucoma medications (P=0.729), and number of previous trabeculectomies (P=0.620) did not show any significant difference between diagnosis. According to tube length, eyes with shorter tube (n=24, <2.7 mm) did not show significant difference in mean age (P=0.428), preoperative IOP (P=0.716), number of preoperative glaucoma medications (P=0.662), and number of previous trabeculectomies (P=0.810) when compared with eyes with longer tube (n=24, longer than 2.7 mm).
The interobserver agreement of the intracameral tube length measurements, T–I distance, and T–C angle were excellent. The ICC was 0.971, 0.925, and 0.953, respectively.
The mean intracameral tube length was 2.93±1.84 mm in all patients (range, 1.12–3.74 mm). The mean length change after 12 months of AGV implantation was −0.20±0.17 (range, −0.54 to 0.20), indicating that the tube length shortened from the initial inserted length. The mean T–I distance was 0.21±0.52 mm (range, 0.11–0.31 mm). The mean change of T–I distance after 12 months of AGV implantation was 0.11±0.07 mm (range, 0.02–0.26 mm). The mean T–C angle was 47.9±5.0° (range, 37.2–49.7°). This changed from −0.2° to −22.3°, with a mean change of −6.7±5.6° (Table 1). The tube position moved forward and rotated to the corneal side, meaning it became closer to the cornea. Serial measurements of the intracameral tube length, T–I distance, and T–C angle are shown in Figure 2. The intracameral tube length (Figure 2a), T–I distance (Figure 2b), and T–C angle (Figure 2c) changed prominently in uveitic glaucoma and glaucoma after penetrating keratoplasty.
The change of intracameral tube length (a), T–I distance (b), and T–C angle (c) after AGV implantation up to 12 months. Note that the changes of the tube parameters are prominent between postoperative 1 month and 3 months. The changes of the tube parameters were significant in uveitic glaucoma (indicated as uveitic) and post penetrating keratoplasty glaucoma (indicated as PPKP).
Representative images of the serial visualization of the AGV tube in cases of uveitic glaucoma are shown in Figure 3. In case a, the tube position did not change significantly; however, the tube length shortened at months 1 (Figure 3a2) and 3 (Figure 3a3) compared with day 1 (Figure 3a1). In case b, the tube length shortened and the tube position moved forward and rotated to face the corneal side at months 1 (Figure 3b2) and 3 (Figure 3b3) compared with day 1 (Figure 3b1). The overlapping of the images at each period shows that the anterior chamber angle and the position of the iris were not changed (Figures 3a4 and b4). However, the tube length shortens in case a, and it shortens and moves forward in case b.
Two representative cases of uveitic glaucoma are shown. In case a, intracameral tube length has shortened with slight rotation of the tube to the corneal side (a1, postoperative day 1; a2, postoperative month 1; and a3, postoperative months 3). In case b, intracameral tube length shortened and the tube position changed to move forward to the corneal side with rotation (b1, postoperative day 1; b2, postoperative month 1; and b3, postoperative months). The images were overlapped to show that the tube changes were independent of the changes of the anterior chamber angle and iris position (a4 and b4, postoperative day 1 in grey; postoperative month 1 in purple red; and postoperative months 3 in green).
Age, gender, lens status, previous trabeculectomy, IOP, and initial intracameral tube length did not show a significant correlation to the changes of intracameral tube length, T–I distance, and T–C angle. However, when patients were divided into subgroups based on their glaucoma diagnosis, uveitic glaucoma and glaucoma after penetrating keratoplasty showed a significant correlation with the intracameral tube length and T–I distance. Uveitic glaucoma was related to changes in tube length (P=0.049) and glaucoma after penetrating keratoplasty was related to changes in tube length (P=0.008) and T–I distance (P=0.001, Table 2).
Discussion
To our knowledge, this study is the first reported to assess serial changes in the AGV tube using AS-OCT. By this serial imaging, we found that the AGV tube tended to shorten and move towards the cornea. This tendency differed significantly according to glaucoma type. In eyes with uveitis and eyes that had previously undergone penetrating keratoplasty, there was a prominent tendency for the AGV tube to shorten and move anteriorly.
Tube malpositioning and corneal contact are common findings in paediatric glaucoma, reported to occur in 25.7–34.8% of cases,8, 9, 10 with one study reporting 11 of 42 eyes (26.2%) requiring surgical intervention for postoperative tube malpositioning.11 It has been reported that tube malpositioning typically occurs anteriorly to the cornea in children after AGV implantation.12 However, tube malposition or tube retraction is not a common finding in AGV implantation in adults. In this regard, tube–corneal contact has been reported to occur in ∼5% of AGV implantations.13, 14 Tube retraction has been reported to occur as a long-term complication, but the rates are very low (reported as 1/3114 and 1/413 patients13). In our study, age was not related to the shortening or anterior migration of the tube, but only one 16-year-old patient was included with the other patients, all ≥20 years of age. Thus, our results may reflect the tendencies of adult patients only. Although tube retraction that requires intervention is not common, we demonstrate that small tube retractions and anterior movements do occur in adults.
There is no specific report showing that uveitic glaucoma and glaucoma after penetrating keratoplasty show more tube-related complications. In the present study, these types of glaucoma showed more prominent tube shortening and migration. Large tube movement has been explained by the loosening of the nonabsorbable suture, a relatively stronger adhesion of the fibrovascular capsule to the fornix, compared with the sclera, tube kinking, or eyeball indentation by the plate.15 However, in this study, the plate was in its original position and the visible extraocular portion of the tube was readily visualized under the conjunctiva, with no obvious kink. The tube length, T–I distance, and T-C angle changed during the first three postoperative months. This is the period when the granulomatous reaction around the tissues of the plate occurs, which is present at the first month and resolves after 4 months.16 The exact reason for the tube migration in the anterior chamber is unknown, but may be partially related to the granulomatous reaction around the plate or a natural tendency of the tube to return to a straight position. In uveitic glaucoma, excessive granulomatous response may occur and peripheral anterior synechia or pre-iris membrane may grow after glaucoma surgery, resulting in increased migration of the tube.17, 18, 19 In glaucoma after penetrating keratoplasty, the change in the structure of the angle and peripheral anterior synechia after penetrating keratoplasty may result in increased migration of the tube.20 However, further study to reveal the exact reason may be needed.
The present study has some limitations. The sample size was restricted and young patients were not included in the study. This means our study group may not precisely represent the general course of AGV implantation. The total postoperative follow-up was relatively short. More longitudinal observation may be needed.
In conclusion, we demonstrate that the length and position of the AGV tube changes after surgery. The change was prominent in uveitic glaucoma and glaucoma after penetrating keratoplasty. Considering longer tube length may minimize complications such as tube retraction in the uveitic glaucoma and glaucoma after penetrating keratoplasty. A customized tube length and position for each patient considering the glaucoma type may have important implications for AGV implantation surgery.

References
Mendrinos E, Dosso A, Sommerhalder J, Shaarawy T . Coupling of HRT II and AS-OCT to evaluate corneal endothelial cell loss and in vivo visualization of the Ahmed glaucoma valve implant. Eye (Lond) 2009; 23 (9): 1836–1844.
Kim CS, Yim JH, Lee EK, Lee NH . Changes in corneal endothelial cell density and morphology after Ahmed glaucoma valve implantation during the first year of follow up. Clin Experiment Ophthalmol 2008; 36 (2): 142–147.
Sarkisian SR, Netland PA . Tube extender for revision of glaucoma drainage implants. J Glaucoma 2007; 16 (7): 637–639.
Syed HM, Law SK, Nam SH, Li G, Caprioli J, Coleman A . Baerveldt-350 implant versus Ahmed valve for refractory glaucoma: a case-controlled comparison. J Glaucoma 2004; 13 (1): 38–45.
Hau S, Scott A, Bunce C, Barton K . Corneal endothelial morphology in eyes implanted with anterior chamber aqueous shunts. Cornea 2011; 30 (1): 50–55.
Sarodia U, Sharkawi E, Hau S, Barton K . Visualization of aqueous shunt position and patency using anterior segment optical coherence tomography. Am J Ophthalmol 2007; 143 (6): 1054–1056.
Park HY, Lee NY, Park CK . Risk factors of shallow anterior chamber other than hypotony after Ahmed glaucoma valve implant. J Glaucoma 2009; 18 (1): 44–48.
Djodeyre MR, Peralta Calvo J, Abelairas Gomez J . Clinical evaluation and risk factors of time to failure of Ahmed Glaucoma Valve implant in pediatric patients. Ophthalmology 2001; 108 (3): 614–620.
O’Malley Schotthoefer E, Yanovitch TL, Freedman SF . Aqueous drainage device surgery in refractory pediatric glaucoma: II. Ocular motility consequences. J AAPOS 2008; 12 (1): 40–45.
Oguz H . Aqueous shunt devices compared with trabeculectomy with mitomycin-C for children in the first two years of life. Am J Ophthalmol 2004; 137 (6): 1163–1164; author reply 1164.
Al-Mobarak F, Khan AO . Complications and 2-year valve survival following Ahmed valve implantation during the first 2 years of life. Br J Ophthalmol 2009; 93 (6): 795–798.
Sarkisian Jr SR . Tube shunt complications and their prevention. Curr Opin Ophthalmol 2009; 20 (2): 126–130.
Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP, Godfrey DG et al. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology 2011; 118 (3): 443–452.
Topouzis F, Coleman AL, Choplin N, Bethlem MM, Hill R, Yu F et al. Follow-up of the original cohort with the Ahmed glaucoma valve implant. Am J Ophthalmol 1999; 128 (2): 198–204.
Law SK, Coleman AL, Caprioli J . Dynamic tube movement of Ahmed glaucoma valve. J Glaucoma 2009; 18 (8): 628–631.
Lloyd MA, Baerveldt G, Nguyen QH, Minckler DS . Long-term histologic studies of the Baerveldt implant in a rabbit model. J Glaucoma 1996; 5 (5): 334–339.
Chang L, Wong T, Ohbayashi M, Bunce C, Barton K, Ono SJ et al. Increased mast cell numbers in the conjunctiva of glaucoma patients: a possible indicator of preoperative glaucoma surgery inflammation. Eye (Lond) 2009; 23 (9): 1859–1865.
Tektas OY, Heinz C, Heiligenhaus A, Hammer CM, Luetjen-Drecoll E . Morphological changes of trabeculectomy specimens in different kinds of uveitic glaucoma. Curr Eye Res 2011; 36 (5): 442–448.
Carreno E, Villaron S, Portero A, Herreras JM, Maquet JA, Calonge M . Surgical outcomes of uveitic glaucoma. J Ophthalmic Inflamm Infect 2011; 1 (2): 43–53.
Karadag O, Kugu S, Erdogan G, Kandemir B, Eraslan Ozdil S, Dogan OK . Incidence of and risk factors for increased intraocular pressure after penetrating keratoplasty. Cornea 2010; 29 (3): 278–282.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lopilly Park, HY., Jung, K. & Park, C. Serial intracameral visualization of the Ahmed glaucoma valve tube by anterior segment optical coherence tomography. Eye 26, 1256–1262 (2012). https://doi.org/10.1038/eye.2012.131
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2012.131