Abstract
Purpose
To assess the efficacy and safety of preoperative intravitreal bevacizumab (IVB) before vitrectomy for diabetic tractional retinal detachment (TRD).
Methods
Using ICD-9 codes, we located all patients with diabetic TRD who underwent 3-port 20-gauge vitrectomy primarily performed by one surgeon between January 2004 and January 2009. Eyes receiving IVB were compared with those not. The following outcomes were compared: visual acuity (VA), duration of surgery, and complication rates.
Results
A total of 99 eyes of 90 patients were included in the analysis. In all, 34 patients received IVB on an average of 11.5 (range, 3–30) days previtrectomy. Age was 46.5 and 51.6 in the IVB and non-IVB groups, respectively. VA was improved significantly in both groups: from 20/617 to 20/62 in the IVB group, and from 20/443 to 20/86 in the non-IVB group (P=0.11 between groups). Operating time and postoperative complications (glaucoma, RD, and revitrectomy rate) were similar in both groups. On comparing IVB and non-IVB eyes in younger patients (≤40), operating time was shorter (P=0.02) and a trend to better VA in the IVB group was seen.
Conclusions
Preoperative IVB may be a useful adjunct to vitrectomy for severe PDR complicated by TRD, particularly in younger diabetics.
Similar content being viewed by others
Introduction
Traditionally, diabetic tractional retinal detachment (TRD) has been associated with severe visual loss. In the Diabetic Retinopathy Vitrectomy Study (DRVS), 70% of eyes had either ‘questionable or definite elevations’ before surgery.1 Approximately 20% of these worsened to no light perception after pars plana vitrectomy (PPV). Since the DRVS, significant improvements in PPV techniques such as endolaser, C3F8 injection, better microscope-viewing systems, and earlier vitrectomy have improved the outcome of diabetic vitrectomy. Mason et al2 reported significant improvement in diabetic vitrectomies conducted in the late 1990's; only 3% progressed to poor visual outcome.
The recent use of preoperative intravitreal injection of bevacizumab (IVB) (Avastin, Genentech Inc., South San Francisco, CA, USA) may potentially further improve diabetic vitrectomy outcome. This vascular endothelial growth factor inhibitor has been reported to decrease intraoperative hemorrhage and facilitate fibrovascular membrane dissection,3,4,5,6,7,8,9,10,11,12 and reduce postoperative vitreous hemorrhage (VH) rates.9,12,13,14 However, these studies were limited by relatively small numbers, heterogeneous retinal pathology (TRD and VH were studied together), and varying surgical techniques (multiple surgeons and multiple gauge vitrectomies). Concern still exists that IVB may worsen TRD,6,15,16,17 and may cause the foveal vascular zone enlargement.18,19 We undertook this study to assess the effect of preoperative IVB on diabetic TRD involving the macula, using a consistent surgical technique (standard 20-gauge PPV primarily performed by one surgeon).
Methods
This retrospective, comparative, and consecutive chart review was approved by the Henry Ford Hospital Institutional Review Board, Detroit, Michigan. Using ICD-9 codes, the charts of all diabetic patients with PDR undergoing PPV primarily performed by one surgeon (URD) between January 2004 and January 2009 at the Henry Ford Hospital were identified and reviewed. Only eyes with TRD involving the macula, with or without a rhegmatogenous component, and with or without VH, were included in the analysis. Eyes with VH dense, enough to prevent visualization of the macula prePPV, were included in the study if macular-involving TRD was seen at surgery. The data were collected from an electronic medical record system that contained scans of handwritten and typed dictations of patient records. Exclusion criteria were previous vitreoretinal surgery except laser, intraocular surgery of any type within the prior 3 months, history of filtering surgery for glaucoma (because of worse visual potential), visual acuity (VA) less than 20/800, which was not explained by VH, and less than 6 months of postoperative follow-up.
Eyes in the bevacizumab group received an injection ≤30 days before surgery. We felt that IVB would cause neovascularization regression as late as 30 days after injection, and that the recurrence of neovascularization would take longer than 30 days. All IVBs were given in a standard manner after obtaining informed consent: 5% betadine, lidocaine subconjunctival injection, and 0.05 ml of bevacizumab (1.25 mg) were used. Anterior chamber paracentesis was not performed. The untreated group consisted of all remaining eyes that did not receive prePPV IVB. Preoperative panretinal photocoagulation (PRP) was attempted in all eyes without prior PRP. Worsening of TRD between the IVB injection and surgery was assessed at the time of surgery by clinical examination.
All PPVs were primarily performed by one surgeon (URD). Fellows performed aspects of the PPVs, but not the membrane dissections. All were standard 20-gauge, 3-port PPVs. After core vitrectomy, the posterior hyaloid was opened and carefully removed as completely as possible. Preretinal fibrovascular tissue and tractional membranes were removed using a combination of segmentation and delamination techniques, primarily with horizontal-cutting scissors. If subretinal fibrotic bands were present, these were removed through a retinotomy with subretinal forceps. The surgical endpoint was relief of traction on the macula and on neovascular fronds that allowed the entire retina to flatten. Hemostasis was maintained by endodiathermy and by prudent elevation of the intraocular pressure. Care was taken not to compromise the intraocular perfusion by high intraocular pressure or low systemic blood pressure. Thorough PRP was administered at 360° extending anterior to the equator in all eyes, regardless of whether the eye had prior PRP. Intraocular retinal tamponade was tailored according to the appearance of the retina after the membranes were removed. If the retina appeared flat, without memory of the removed membrane, no tamponade or air was used. SF6 and C3F8 were used if significant residual retinal folds remained or if a retinal break occurred. Silicone oil was injected if retinectomy was performed or if multiple inadvertent retinal breaks occurred. Cryotherapy was not used, and no supplemental PRP was given in the 6-month postoperative period.
We compared the bevacizumab group to the non-bevacizumab group for the entire cohort, and after stratifying into two age groups (older than 40; 40 and under). This age cutoff was selected because of our clinical impression that patients older than 40 appear to have less vascular and more fibrotic membranes. Primary outcomes of this study were best VA at 6 months or later, operating time and the incidence of postoperative complications (neovascular glaucoma, RD, and additional vitreoretinal surgery (for RD or VH)).
Descriptive statistics including mean and standard deviation were calculated for case characteristics. Student's t-test for unequal variance was used for comparing means. Fisher exact test was used for comparing categorical variables. Statistical significance was considered as <0.05. Snellen VA was converted to logarithm of minimum angle of resolution (logMAR) units for purposes of analysis. Counting fingers at 2 feet was assigned a VA of 20/2000, and hand motion at 2 feet was converted to a VA of 20/20 000; as recommended by Holladay.20
Results
For the 5-year study period, we retrieved 312 PPVs performed in patients with PDR. Of these 124 had TRD involving the macula. Of these 24 were excluded because of a history of filtering surgery for glaucoma (2 eyes), minimal visual potential (3 eyes), or less than 6 months of postPPV follow-up (20 eyes, 12 due to patient death). Of the 99 patients analyzed, 34 received prePPV IVB and 65 did not. Of 69, 20 (29%) eyes of patients older than 40 received IVB, and 14 of 30 (47%) eyes of patients ≤40 received IVB (P=0.11). The average time between injection and PPV in our 34 study patients was 11.5±7 (range, 3–30) days.
Patient demographics and the baseline ocular findings are shown in Table 1. Patients had poor diabetic control, with an average HbA1c of 9.0 and 90% of patients had systemic hypertension. Table 2 shows the outcome measures. Figures 1 and 2 show the pre- and postoperative VA for the over 40-year-old patients, and for the 40 and under patients, respectively. Figure 3 shows the retina of a young patient from the IVB group pre- and postPPV.
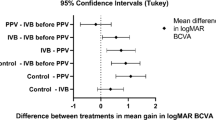
The postoperative VA was significantly better than the preoperative (P<0.01) in all analysis groups. LogMAR change ±SD for IVB and non-IVB eyes of the total cohort was 1.00±0.9 and 0.69±1.1 (P=0.13), respectively. Operating time comparison for the total cohort was 105.5±29.9 and 104.9±41.5 (P=0.90), respectively.
No preoperative injection-related complications were seen, except for minor worsening of the TRD in three IVB eyes. No IVB eye developed obvious rhegmatogenous RD between the IVB injection and PPV. Six eyes of the non-IVB group had preoperative iris neovascularization. Two of these lost seven or more lines of VA, three eyes gained eight or more lines, and one eye's VA remained unchanged postPPV. Only one eye had progression of neovascular glaucoma after surgery.
Discussion
Our results show a significant improvement in VA in both the bevacizumab and non- bevacizumab groups. This, as others have suggested,3 is most likely due to improved vitrectomy techniques. Although the improvement in VA was not significantly greater in the IVB group, the IVB eyes had a possible trend towards better VA. Further, the younger patients that received IVB had the largest improvement in VA, the best postoperative VA and no postoperative VA loss (Figure 2). Although surgical time comparison for IVB and non-IVB groups was similar for the total cohort (possibly due to resident and fellow teaching confounding the true surgery time), it was significantly shorter for the younger IVB patients. These two findings suggest that preoperative IVB made TRD surgery more efficacious, especially in younger diabetic patients. This corroborates the finding of Yeoh et al5 that IVB was most efficacious in eyes with active retinal neovascularization.
The following factors may explain why the young diabetics of our study appeared to gain the most from preoperative IVB. First, younger patients have more vascular preretinal membranes and intraoperative bleeding is therefore more problematic. Intraoperative bleeding in turn is associated with poorer visualization and therefore a higher incidence of surgical complications such as retinal tears. IVB causes regression of neovascularization which decreases intraoperative bleeding and facilitates membrane peeling and delamination.12 Second, younger patients tend to have less macular ischemia and therefore more visual potential. And third, the lenses of younger patients are less prone to develop postvitrectomy cataracts.
Many have reported that IVB worsens TRD.6,15,16,17 Three IVB study eyes had minor TRD worsening and none had developed rhegmatogenous RD between IVB injection and PPV. We may have missed some TRD worsening as our patients did not undergo a full retinal evaluation between IVB injection and PPV, eyes with dense VH were not assessed for worsening of their TRD by ultrasound preoperatively, and eyes with clear media did not undergo optical coherence tomography between IVB injection and PPV. Nevertheless, only one eye of the 34 that received IVB lost VA (Figure 1), and this was due to postoperative open-angle glaucoma. We therefore feel that this potential complication of preoperative IVB, even if it did occur, was offset by what may be safer and more efficient surgery. Although we saw little worsening of the TRD due to IVB with a mean time between injection and PPV of 11.5 days, we nevertheless prefer to limit IVB to 2–10 days before surgery. Di Lauro's12 recent study showed that the effect of IVB was efficacious at both 7 and 20 days prePPV, with their 7-day group having slightly better outcomes. Further study on both the timing and dose of preoperative IVB is necessary. Until there are more data on this question, we advise not to inject IVB in TRD patients who have not been scheduled for surgery or who are awaiting medical clearance. Many diabetic TRD patients have renal, cardiac, and hypertensive disease, which may delay their surgery.
No significant differences between silicone oil use and the repeat surgery rates were seen between IVB and non-IVB eyes. Both silicone oil use and postoperative rhegmatogenous RD were relatively rare, which we attribute to the improvements in surgical technique since the DRVS. Regarding recurrent VH; although we did not see a significant difference between the IVB and non-IVB groups, others have reported that preoperative IVB did lower the incidence of postoperative VH.7,9,12,13,14
The main limitation of our study was the selection of patients for preoperative IVB. Initially, in 2006, when we started using this adjunct for PPV, there was a selection bias for using IVB in eyes with more vascular membranes. This bias was relevant for the first three IVB study eyes, and may explain why more of the younger patients received IVB (47 vs 29%), before we decided to use preoperative IVB in all TRD patients. Nevertheless, we feel this did not significantly impact on our results, as within a few surgeries we decided to use IVB preoperatively for all diabetic TRDs. Furthermore, if this selection bias was significant, it is likely that the more difficult eyes would have received IVB, which would bias the IVB eyes to poorer outcomes. This was not the case. Outcome measures were as good or better in the IVB eyes. Although most non-IVB eyes underwent surgery between 2004 and 2006, and most IVB eyes had their surgery between 2006 and January 2009, there was no significant change in equipment or technique over these 5 years.
We used the total surgical time, even though the duration of elevated intraoperative IOP and the number of times endodiathermy was used for hemostasis are better measures of the intraoperative benefit of prePPV IVB. Because of the retrospective nature of our study, we were unable to retrieve the latter intraoperative data. Although the surgical time analysis may have been limited by fellow's involvement in the case, we feel that this outcome measure is nevertheless relevant as all membrane dissections were primarily performed by the same surgeon (URD). Since the IVB eyes underwent their surgeries during 2006–2008, and the non-IVB eyes earlier (2004–2006), it is possible that the learning curve of the primary surgeon had a role in the slightly better outcomes of the IVB eyes. We do not believe that the learning curve was significant, as our primary surgeon had more than 12 years of experience with these surgeries before 2004.
Other limitations of our study are that the duration of the macular TRD could not be accurately analyzed retrospectively, that lens status was not controlled for (even though 51% of eyes had gas tamponade), and that follow-up was limited to 6 months in some eyes and longer in others.
Our study does have the following strengths: consecutive collection of data, relatively homogeneous group of severe diabetic patients (Table 1), IVB and non-IVB groups were balanced preoperatively (Table 1), standardized surgical technique (one center, consistent primary surgeon, consistent equipment and a similar technique), and the all patients who were studied had advanced PDR with TRD involving the macula. Eyes with VH without fibrotic membranes involving the macula were excluded from our study. Although our study was unable to show clear statistic benefit for preoperative IVB for diabetic TRD, our data do show that preoperative IVB appears to be safe in patients of any age, and may contribute to a better outcome in younger diabetic patients.

References
Diabetic Retinopathy Vitrectomy Study Research Group. Two-year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic Retinopathy Vitrectomy Study Report #1. Ophthalmology 1985; 92: 492–502.
Mason JO, Colagross CT, Haleman T, Fuller JJ, White MF, Feist RM et al. Visual outcome and risk factors for light perception and no light perception vision after vitrectomy for diabetic retinopathy. Am J Ophthalmol 2005; 140: 231–235.
Chen E, Park CH . Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006; 26: 699–700.
Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, Miniaci S, Williams G . Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol 2008; 246: 837–842.
Yeoh J, Williams C, Allen P, Buttery R, Chiu D, Clark B et al. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Clin Experiment Ophthalmol 2008; 36: 449–454.
Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009; 116: 927–938.
Lo WR, Kim SJ, Aaberg Sr TM, Bergstrom C, Srivastava SK, Yan J et al. Visual outcomes and incidence of recurrent vitreous hemorrhage after vitrectomy in diabetic eyes pretreated with bevacizumab (Avastin). Retina 2009; 29: 926–931.
da R Lucena D, Ribeiro JA, Costa RA, Barbosa JC, Scott IU, de Figueiredo-Pontes LL et al. Intraoperative bleeding during vitrectomy for diabetic tractional retinal detachment with versus without preoperative intravitreal bevacizumab (IBeTra study). Br J Ophthalmol 2009; 93: 688–691.
Romano MR, Gibran SK, Marticorena J, Wong D, Heimann H . Can a preoperative bevacizumab injection prevent recurrent postvitrectomy diabetic vitreous haemorrhage? Eye 2009; 23: 1698–1701.
Ishikawa K, Honda S, Tsukahara Y, Negi A . Preferable use of intravitreal bevacizumab as a pretreatment of vitrectomy for severe proliferative diabetic retinopathy. Eye 2009; 23: 108–111.
Modarres M, Nazari H, Falavarjani KG, Naseripour M, Hashemi M, Parvaresh MM . Intravitreal injection of bevacizumab before vitrectomy for proliferative diabetic retinopathy. Eur J Ophthalmol 2009; 19: 848–852.
di Lauro R, De Ruggiero P, di Lauro R, di Lauro MT, Romano MR . Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2010; 248: 785–791.
Yang CM, Yeh PT, Yang CH, Chen MS . Bevacizumab pretreatment and long-acting gas infusion on vitreous clear-up after diabetic vitrectomy. Am J Ophthalmol 2008; 146: 211–217.
Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R . Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients a randomized clinical trial. Ophthalmology 2009; 116: 1943–1948.
Arevalo JF, Maia M, Flynn Jr HW, Saravia M, Avery RL, Wu L et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 2008; 92: 213–216.
Moradian S, Ahmadieh H, Malihi M, Soheilian M, Dehghan MH, Azarmina M . Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2008; 246: 1699–1705.
Jonas JB, Schmidbauer M, Rensch F . Progression of tractional retinal detachment following intravitreal bevacizumab. Acta Ophthalmol 2009; 87: 571–572.
Lee SJ, Koh HJ . Enlargement of the foveal avascular zone in diabetic retinopathy after adjunctive intravitreal bevacizumab (Avastin) with pars plana vitrectomy. J Ocul Pharmacol Ther 2009; 25: 173–174.
Chen E, Hsu J, Park CH . Acute visual acuity loss following intravitreal bevacizumab for diabetic macular edema. Ophthalmic Surg Lasers Imaging 2009; 40: 68–70.
Holladay JT . Visual acuity measurements. J Cataract Refract Surg 2004; 30: 287–290.
Acknowledgements
Russell Pokroy received fellowship grants from the American Physicians Fellowship for Medicine in Israel, and from the Israel Ophthalmic Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Meeting presentations: Presented in part at the Retina Congress 2009, New York, NY, October 2009.
Rights and permissions
About this article
Cite this article
Pokroy, R., Desai, U., Du, E. et al. Bevacizumab prior to vitrectomy for diabetic traction retinal detachment. Eye 25, 989–997 (2011). https://doi.org/10.1038/eye.2011.149
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2011.149
Keywords
This article is cited by
-
Pre-operative intravitreal bevacizumab for tractional retinal detachment secondary to proliferative diabetic retinopathy: the Alvaro Rodriguez lecture 2023
International Journal of Retina and Vitreous (2023)
-
Approaches to the Repair of Diabetic Traction Retinal Detachments
Current Surgery Reports (2022)
-
Sickle cell retinopathy. A focused review
Graefe's Archive for Clinical and Experimental Ophthalmology (2019)
-
Bevacizumab as an adjunct to vitreoretinal surgery for diabetic retinopathy in East Africa
Eye (2013)
-
Six-month visual outcome after pars plana vitrectomy in proliferative diabetic retinopathy with or without a single preoperative injection of intravitreal bevacizumab
International Ophthalmology (2012)