Abstract
Purpose
To establish the antioxidant status of the aqueous humour in glaucoma associated with exfoliation syndrome (XFG) and to compare it to primary open-angle glaucoma (POAG) and cataract patients.
Methods
Patients were diagnosed with POAG, XFG, or cataract (n=25 for each group). Total reactive antioxidant potential (TRAP) was measured by chemiluminescence. Ascorbic acid levels and the activities of catalase, glutathione peroxidase (GPx), and superoxide dismutase (SOD) were measured spectrophotometrically.
Results
TRAP value was lower in XFG (28±2 μM Trolox) than in POAG (55±8 μM Trolox; P<0.001). TRAP values in both glaucomas were lower than the cataract value (124±5 μM Trolox; P<0.001). A decrease in ascorbic acid was measured in XFG (230±20 μM) compared with POAG (415±17 μM; P<0.001). Ascorbic acid in both glaucomas was lower than in cataract (720±30 μM; P<0.001). A significant increase in GPx was found in XFG (30±2 U/ml) compared with POAG (16±3 U/ml). GPx activity in both glaucomas was increased when compared with cataracts (6±2 U/ml; P<0.001). A significant increase of 67% in SOD activity was observed in the glaucoma group vscataract group (27±3 U/ml; P<0.001), but no changes were found between both glaucomas.
Conclusions
The antioxidant status of the aqueous humour may play a role in the pathophysiology of both glaucomas.
Similar content being viewed by others
Introduction
Exfoliation syndrome (XFS) is the most common cause of secondary open-angle glaucoma.1 It is a generalized age-related disorder of the extracellular matrix with abnormalities in the basal membranes.2
The clinical diagnosis is made by the presence of exfoliative material on the surface of the anterior capsule of the lens. Exfoliative material may also be present in the corneal endothelium and the trabecular meshwork. Other clinical features include atrophy of the pupillary border and iris transillumination defects.3
Exfoliation syndrome may be associated with ocular problems such as high intraocular pressure (IOP) and glaucomatous optic neuropathy. It may also be associated with poor mydriasis, zonular instability, corneal endotheliopathy, central retinal vein occlusion, and cataract.2, 3 Systemic associations found in XFS include angina pectoris, hypertension, myocardial infarction, and stroke.4
The exact aetiology and pathogenesis are unknown. The most accepted theory postulates that it is an age-related process of build-up of an abnormal elastotic material.5
The principal ocular cells implicated in the production of exfoliative material are those closely associated with the aqueous humour circulation, and they are influenced by the substances present in it. An investigation of the qualitative and quantitative alterations of the aqueous humour composition might, therefore, provide an important insight into the factors involved in this disorder.
Recent studies reported differences in the concentration of matrix metalloproteinases and growth factors in the aqueous humour of XFS patients. It is well known that growth factors and proteases can be activated by free radicals, so the occurrence of oxidative stress and therefore the antioxidant status of the aqueous humour may play a role in the oxidative metabolism of the cells implicated in the production of exfoliative material.6, 7, 8
Oxidative stress can be defined as an increase in the intracellular concentrations of reactive oxygen species (ROS) over physiological values.9 This situation may be reflected by changes in the antioxidant defences that can be depleted because of the ROS action or increased as a protective response.10
The aqueous humour contains a variety of enzymatic and non-enzymatic antioxidant defences. Low molecular weight antioxidants, such as glutathione (GSH), ascorbic acid, cysteine, and tyrosine, have been identified in the aqueous humour.11 Ascorbic acid, a molecule capable of scavenging superoxide anion, hydroxyl radical, and singlet oxygen,12, 13 is present at high concentrations in aqueous humour (1–2 mM).14, 15 Increasing evidence suggests that ascorbic acid plays an important role in the defence mechanisms of the ocular tissues against free radical damage.16, 17 A decrease in ascorbic acid and an increase of 8-isoprostaglandin F2a, a free radical catalysed arachidonic acid product used as an in vivo oxidative stress marker, have been reported in the aqueous humour of patients with XFS.18 Low molecular weight antioxidants can be determined by total reactive antioxidant potential (TRAP). This is a measurement of the ability of a compound to reduce pro-oxidants or reactive species of pathologic significance. TRAP values of biological samples can be determined through a chemiluminescence method, which allows a precise evaluation over short periods of time.19, 20
Antioxidants in the aqueous humour also include enzymatic systems such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx).21, 22, 23 The formation of highly cytotoxic oxygen-derived free radicals is prevented by these enzymes.
The purpose of this study is to evaluate the antioxidant status of the aqueous humour in patients with glaucoma associated with XFG and to compare it with primary open-angle glaucoma (POAG) and cataract patients.
Patients and methods
Patients
The patients were divided into three groups: patients with XFG, POAG, and cataract. Each group consisted of 25 patients. All patients were Caucasians and matched for age and sex. There were no statistically significant differences between the groups in terms of age and sex. Age was 73±2 years old for the XFG group, 70±10 years old for the POAG group, and 73±2 years old for the cataract group.
Preoperative IOP was 25±5 mmHg for the XFG patients, 26±4 mmHg for the POAG group, and 14±6 mmHg for the cataract group.
Vertical cup/disc ratio was 0.89±0.01 for XFG patients and 0.89±0.01 for the POAG group.
Inclusion criteria
Glaucoma patients included in the study had a diagnosis of POAG or XFG. Structural definition: vertical cup-to-disc ratios (C/D) of 0.7 or more, asymmetry in the C/D of 0.2 or more and/or thinning of the neuroretinal rim-to-disc ratio of less than 0.1 with corresponding perimetric damage. The Disc Damage Likelihood Scale system was used to evaluate the rim-to-disc ratio.24, 25 Functional definition: glaucoma hemifield test outside normal limits, and three adjacent points in the 5% level on the pattern deviation plot, using the 24–2 strategy of the Humphrey perimeter.24, 25 Visual fields were considered reliable if false-negative and false-positive responses were below 33%. Unreliable visual fields were repeated on the same day. If the second visual field was also unreliable, inclusion was made only on the basis of structural damage.
All individuals had advanced glaucoma and elevated IOP despite the use of maximum tolerated medical therapy, and were scheduled for trabeculectomy. Patients with glaucoma (XFG and POAG) were using a variety of topical antiglaucoma medications. Maximal tolerated medical therapy usually included a combination of timolol-dorzolamide (or timolol and brimonidine), although we have eliminated patients on prostaglandin analogues. Antiglaucoma medications were not stopped before the procedures.
Patients enrolled in the cataract group had senile cataract. Cataract patients received topical phenylephrine and tropicamide as dilating drops before surgery. Non-steroidal anti-inflammatory agents were not administered before the procedure. All patients in this group did not have glaucoma. In all cases, this was the first intraocular surgical procedure.
All patients underwent a complete ophthalmic examination. This included an anamnesis, best corrected visual acuity, slit-lamp examination, Goldmann applanation tonometry, and fundus examination with a dilated pupil. Gonioscopy was performed in all cases with a four-mirror goniolens. All patients (POAG and XFG) had an open angle (grade 3 or 4 of the Shaffer classification). The optic nerve was evaluated with a 78 dioptre lens, and the vertical and horizontal C/D ratios were recorded, as well as the presence of any notch or haemorrhage, and the appearance of the neuroretinal rim. Computerized perimetry was performed with the Humphrey 750, threshold strategy 24–2, or similar program with the Octopus.
In all glaucoma patients, no other explanation for the optic nerve damage and the visual field loss should be found apart from the glaucoma.
Exfoliation syndrome was defined by the presence of exfoliation material on the anterior surface of the lens. Exfoliation material was also investigated in the pupillary border, corneal endothelium, anterior hyaloid, and angle. However, for the diagnosis of XFS, only the presence of material in the anterior surface of the lens was considered. This surface, with the pupil dilated, was carefully examined for the presence of exfoliative material, using the high magnification of the slit-lamp and adequate illumination.
Exclusion criteria
We excluded patients with previous intraocular surgeries, laser treatment, uveitis, any posterior segment pathologies, diabetes mellitus, or any other systemic disease that may have influenced our measurements. We excluded from this study patients with other ophthalmic conditions such as angle closure, low tension, congenital glaucoma, trauma, or pigment dispersion syndrome.
We have eliminated patients on prostaglandin analogue treatment. None of the patients smoked, had special diets, or were taking antioxidant vitamins such as α-tocopherol, ascorbic acid, or non-steroidal anti-inflammatory agents. The only systemic medications allowed were those for blood hypertension.
Aqueous humour sampling
Aqueous humour (0.1–0.2 ml) was rapidly and carefully collected at the beginning of the surgery through a paracentesis, using a 27-gauge needle connected to a tuberculin syringe under an operating microscope. Aqueous humour was immediately cooled at −70°C and transported to the laboratory to run all the assays. All the samples were protected from light. Samples were evaluated as soon as possible during the first 24 h after the surgery.
Biochemical determinations of non-enzymatic antioxidants
Ascorbic acid
Ascorbic acid concentration in the aqueous humour was evaluated spectrophotometrically according to the method described by Kyaw.26 Samples were treated with phosphotungstic acid (1.36 M) and incubated at room temperature for 30 min. After centrifugation, the absorbance of the supernatant was measured at 700 nm. A standard curve was established with a set of serial dilutions of ascorbic acid. Results were expressed as μM.
TRAP
Total reactive antioxidant potential was measured by chemiluminescence in a Luminoskan V 1.2-0 liquid scintillation counter. The reaction medium consisted of 20 mM 2,2-Azobis (2-amidinopropane) (ABAP) and 40 μM luminol. The system was calibrated with different concentrations of Trolox (0.25–0.50 μM). A comparison of the induction time after the addition of Trolox and the aqueous humour allows for calculation of the total antioxidant capacity (TRAP) as the equivalent of Trolox concentration necessary to produce the same induction time.20, 27
Biochemical determinations of the antioxidant enzymes
GPx activity
Glutathione peroxidase activity was determined by following reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidation at 340 nm. The reaction medium consisted of 100 mM phosphate buffer (pH 7.50) in the presence of 10 μM reduced glutathione, 6 U/ml glutathione reductase, and 10 mM tert-butyl hydroperoxide. Results were expressed as U/ml of aqueous humour. One unit corresponds to 1 μmol NADPH oxidation per minute per ml of aqueous humour.28
Catalase levels
Catalase activity was determined by measuring the decrease in absorption at 240 nm in a reaction medium consisting of 100 mM phosphate buffer (pH 7.20) and 10 mM hydrogen peroxide (H2O2). The results were expressed as catalase content in fmol/ml of aqueous humour.29
SOD activity
Superoxide dismutase activity was determined in aqueous humour samples by measuring the inhibition of the rate of the autocatalytic adenochrome formation at 480 nm in a reaction medium containing 1 mM epinephrine and 50 mM glycine/NaOH (pH=10.20). The enzyme activity was expressed as SOD U/ml of aqueous humour. One unit is defined as the amount of enzyme that inhibits the rate of adrenochrome formation by 50%.30
Chemicals
All the chemicals were purchased from Sigma Aldrich Chemical (St Louis, MO, USA).
Data and statistical analysis
Statistical calculations were performed with the GraphPad InStat statistical package for Windows. Data are expressed as the mean value±SEM (standard error of the mean). The statistical significance of the differences between the XFG and POAG groups was calculated by the two-tailed unpaired Student's t-test, and a probability value of P smaller than 0.01 indicated a statistically significant difference.
Statement of ethics
We certify that institutional regulations concerning the ethical use of human volunteers were followed during this research.
This study was approved by the Human Subjects Committee of the University of Buenos Aires, and adhered to the Declaration of Helsinki.
Written informed consent was obtained from all participants.
Results
A total of 75 aqueous humour samples were analysed, 25 for each of the following groups: patients with XFG, POAG and cataract. Aqueous humour samples were compared regarding the alterations in the levels of non-enzymatic antioxidants with two parameters: ascorbic acid concentration and the TRAP. The activity of the antioxidant enzymes GPx, catalase, and SOD was also measured.
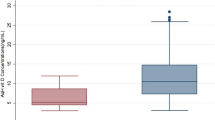
The ascorbic acid concentration in the aqueous humour was 230±20 μM for the XFG group, which represents a 45% decrease when compared with the POAG group. The mean value of the POAG group was 415±17 μM (P<0.001). Ascorbic acid levels of both glaucomas were lower than in the cataract group (720±30 μM; P<0.001) (Figure 1).
Ascorbic acid concentration from the aqueous humour in patients with glaucoma associated with exfoliation syndrome (XFG), compared with primary open-angle glaucoma (POAG) and cataract patients. The values are represented as mean±SEM for 25 XFG patients, 25 POAG patients, and 25 cataract patients. *P<0.001.
On the other hand, the mean values of the TRAP were found to be 55±8 μM Trolox in the aqueous humour of the POAG group and 28±2 μM Trolox in the aqueous humour of the XFG group. In other words, the levels of TRAP were significantly decreased (49%) in the aqueous humour of the XFG group when compared with that of the POAG group (P<0.001). TRAP values of both glaucomas were lower than the cataract value (124±5 μM Trolox; P<0.001) (Figure 2).
Total reactive antioxidant potential from the aqueous humour in patients with glaucoma associated with exfoliation syndrome (XFG), compared with primary open-angle glaucoma (POAG) and cataract patients. The values are represented as mean±SEM for 25 XFG patients, 25 POAG patients, and 25 cataract patients. *P<0.001.
The activity of GPx, catalase (CAT) levels, and SOD activity were determined in the aqueous humour of the XFG group and compared with those measured in the POAG and cataract groups. These data are summarized in Table 1.
A significant increase in GPx activity was found in the aqueous humour of the XFG group when compared with that of the POAG group, whereas no significant changes were found in the CAT levels and SOD activity. GPx activity in the aqueous humour of the XFG patients showed an 87% increase when compared with the POAG group. The mean value of GPx in the aqueous humour from the XFG group was 30±2 U/ml, and for the POAG group it was 16±3 U/ml (P<0.001). GPx of both glaucomas showed an increase when compared with the cataract group (6±2 U/ml; P<0.001).
On the other hand, no significant changes were found in catalase activity. Catalase levels in the aqueous humour from the XFG and POAG groups showed mean values of 40±5 and 42±4 fmol/ml, respectively, whereas in the cataract patients it was 38±7 fmol/ml.
The mean value of SOD in the aqueous humour of the XFG group was 44±7 vs 42±5 U SOD per ml in the POAG group. A significant increase of 67% in the SOD activity was observed in both glaucoma groups vs the cataract group (27±3 U/ml; P<0.001), but no changes were found between both glaucomas.
Discussion
The results of the present study suggest that alterations of the antioxidant defence system are present in the aqueous humour from patients with glaucoma associated with XFG as compared with POAG and cataract patients. To our knowledge, this is the first report that establishes changes in the antioxidant defence system in the aqueous humour of patients with XFG or POAG without cataract. Earlier works have reported alterations in the levels of antioxidants of aqueous humour of patients scheduled for phacoemulsification surgery with diagnoses of pseudoexfoliation syndrome or with coexistent cataract and glaucoma.31, 32
The ascorbic acid levels in XFG patients were decreased 45% compared with POAG patients. These findings seem to be significant because ascorbic acid is essential in cells for its antioxidant capacity and its role in regenerating vitamin E and glutathione.33 In addition to the protective role of ascorbic acid against free radical damage, it also modulates the synthesis of various extracellular matrix molecules such as collagen, elastin, laminin, and glycosaminoglycans.34 Thus, alterations in ascorbic acid concentrations may produce an increase in the fibrillar material deposits that affect the normal aqueous flow through the trabecular meshwork. Significantly reduced levels of ascorbic acid have been reported in the aqueous humour of exfoliation patients with cataract, suggesting a faulty antioxidant defence system.35 Our results are in accordance with these previously published values despite the fact that our exfoliation patients were also diagnosed with glaucoma.
In addition to the decrease in ascorbic acid levels, a significant decay in the aqueous humour TRAP level of the XFG group was found when compared with the POAG group. These results indicate a significant reduction in the concentrations of water-soluble antioxidants in the aqueous humour, such as glutathione, ascorbic acid, tyrosine, and cysteine. Homocysteine is a non-essential sulphur amino acid produced as an intermediate in the metabolism of cysteine. In recent studies, the role of homocysteine in the development of exfoliation glaucoma was investigated.36 The toxicity mechanism of homocysteine may be because of its oxidation in the presence of transition metals, generating superoxide anion, H2O2, hydroxyl radical, and sulphurated radicals.9 Homocysteine was found to be elevated in the aqueous humour and the plasma of patients with XFG coexistent with cataract and normal IOP.37, 38 Moreover, low levels of glutathione were found in the aqueous humour of patients with XFG coexisting with cataract and normal IOP.39
The changes measured in our work in non-enzymatic antioxidants may be because of the occurrence of oxidative stress in the XFG eye that makes the organ more susceptible than the POAG eye to the damage associated with ROS production. In such conditions of deficient antioxidant non-enzymatic defences, an increase in the activity of antioxidant enzymes could be expected as a tissue adaptative response. According to this, the XFG group presented a higher GPx activity in the aqueous humour when compared with the POAG group.
Alterations in GPx activity might have important consequences on the steady state concentration of H2O2. The aqueous humour normally contains H2O2, which is toxic to the cells. Nevertheless, H2O2 is an essential component of several signal-transduction pathways,40 but when its levels exceed the physiological values, it is removed by the two antioxidant enzymes, catalase and GPx. As GPx removes H2O2 using glutathione as a cofactor, this might contribute to the depletion of glutathione. Glutathione is involved in ascorbic acid metabolism, and its depletion produces ascorbyl radicals that cannot be regenerated to ascorbic acid.9
Our results are consistent with this mechanism, as an increase in the activity of GPx and a decrease of the TRAP and ascorbic acid concentration were measured, providing evidence for a role of free radicals-induced oxidative damage in XFG. We suggest that this situation may have been because of a defective redox cycling of reduced/oxidized glutathione.
No significant changes were found in the catalase levels, and this may be due to the nitric oxide (NO) inhibition of the catalase because of the union of NO to the haem group of the enzyme.41 NO competes with H2O2 for the union to the complex I of the catalase; when this enzyme is inhibited, H2O2 has to be metabolized by GPx. GPx activity was found increased and acts as a compensatory mechanism to ameliorate the oxidative stress in the aqueous humour of XFG and POAG patients. Nevertheless, in the present study, we did not find any statistically significant differences in SOD activity between the XFG and POAG groups.
It is better to perform oxidative stress assays on glaucomatous aqueous humour samples that have not received topical medication. As far as we know, there are no studies that have evaluated oxidative stress parameters in the aqueous humour of glaucomatous eye samples that have not received topical medication for a reasonable time before sampling. Another issue to be considered is how many suitable patients in clinical practice undergo antiglaucomatous surgery without any antiglaucomatous instillation. It is not precisely known what effect local medication could have on our results. We did not find any changes when we tested the effect of these medications in TRAP values and antioxidant enzyme activities in vitro.20
In the eye, an altered oxidant/antioxidant balance may result in a number of molecular changes that contribute to the development of age-related ocular diseases such as macular degeneration and glaucoma.42, 43, 44, 45, 46 It has been suggested that the presence of a chronic oxidative stress situation induced by free radicals can compromise the trabecular meshwork function.47, 48 Our results indicate that in both glaucoma groups, the activity of SOD is increased when compared with the cataract group. A recent study found an increased activity of this enzyme in patients with cataract associated with XFG vs non-exfoliative cataract patients.49
Ischaemia produced by changes in IOP and the resultant decrease of oxygen flow into the tissue may lead to an increase in intracellular calcium ions concentration, which may activate proteolysis and lead to the conversion of xanthine reductase into xanthine oxidase, which produces superoxide anion and a compensatory increase in SOD activity.9, 50
If oxygen active species are implicated in trabecular meshwork cell damage, an associated antioxidant mechanism should be present to repair the oxidative injury. The defence against the harmful effects of free radicals is achieved by endogenous antioxidant compounds and antioxidant enzymes present in the aqueous humour. The antioxidant status of aqueous humour might therefore provide an important insight into the factors involved in the development of glaucoma.
The changes observed in the antioxidant defences in the aqueous humour suggest a possible role for the active oxygen species in the tissue damage and pathogenesis of XFG. Our findings suggest that free radicals action with a depleted endogenous non-enzymatic defence system play a role in the worse damage observed in XFG eyes than in POAG eyes. At any level of IOP, damage in XFG is worse than in POAG, prognosis is also worse, progression is more rapid, response to medical therapy is worse, needs surgery more often, and blinds more people. Our results would suggest that further research regarding the use of antioxidants as an adjunct therapy in glaucoma may be indicated.
References
Ritch R . Exfoliation syndrome: the most common identifiable cause of open-angle glaucoma. J Glaucoma 1994; 3: 176–178.
Ritch R, Schlötzer-Schrehardt U . Exfoliation syndrome. Surv Ophthalmol 2001; 45: 265–315.
Naumann G, Schlötzer-Schrehardt U, Küchle M . Pseudoexfoliation syndrome for the comprehensive ophthalmologist. Intraocular and systemic manifestations. Ophthalmol 1998; 105: 951–968.
Schlötzer-Schrehardt U, Koca MR, Naumann G . Pseudoexfoliation syndrome. Ocular manifestation of a systemic disorder? Arch Ophthalmol 1992; 110: 1752–1756.
Schlötzer-Schrehardt U, Naumann G . Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol 2006; 141: 921–937.
Koliakos G, Schöltzer-Schrehardt U, Konstas AG, Bufidis T, Georgiadis N, Dimitriadou A . Transforming and insulin-like growth factors in the aqueous humor of patients with exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol 2001; 239: 482–487.
Schöltzer-Schrehardt U, Lommatzsch J, Küchle M, Konstas A, Naumann G . Matrix metalloproteinases and their inhibitors in aqueous humor of patients with exfoliation syndrome glaucoma and primary open-angle glaucoma. Invest Ophthalmol Vis Sci 2003; 44: 1117–1125.
Koliakos G, Konstas A, Triantos A, Ritch R . Increased growth factor activity in the aqueous humor of patients with exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol 2000; 238: 491–495.
Halliwell B, Gutteridge J . Free Radicals in Biology and Medicine, 3rd ed. Oxford University Press Inc: Oxford, UK, 1989.
Sies H . Oxidative Stress. Academic Press: San Diego, 1985.
Richer SP, Rose RC . Water soluble antioxidants in mammalian aqueous humor. Interaction with UV and hydrogen peroxide. Vis Res 1998; 38: 2881–2888.
Tso M . Retinal photic injury in normal and scorbutic monkeys. Trans Am Ophthalmol Soc 1987; 85: 498–556.
Rose R, Bode A . Ocular ascorbate transport and metabolism. Comp Biochem Pharm 1991; 100: 273–285.
Garland D . Ascorbic acid and the eye. Am J Clin Nutr 1991; 54: 1193–1202.
Varma SD . Ascorbic acid and the eye with special reference to the lens. NY Acad Sci 1987; 498: 280–306.
Varma SD . Scientific basis for medical therapy of cataracts by antioxidants. Am J Clin Nutr 1991; 53: 335–345.
Reddy VN, Giblin FJ, Lin LR, Chakrapani B . The effect of aqueous humor ascorbate on ultraviolet-B induced DNA damage in lens epithelium. Invest Ophthalm Vis Sci 1998; 39: 344–350.
Koliakos G, Konstas A, Schlötzer-Schrehardt U, Hollo G, Katsimbris I, Georgiadis N et al. 8-isoprostaglandin F2a and ascorbic acid concentration in the aqueous humor of patients with exfoliation syndrome. Br J Ophthalmol 2003; 87: 353–356.
Evelson P, Travacio M, Repetto M, Escobar M, Llesuy S, Lissi E . Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 2001; 388: 261–266.
Ferreira S, Lerner F, Brunzini R, Evelson P, Llesuy S . Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol 2004; 137: 62–69.
Behnding A, Svensson B, Marklund S, Karisson K . Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sci 1998; 39: 471–475.
Cotagliola C, Iuliano G, Menzione M, Rinaldi E, Vito P, Auricchio G . Effect of vitamin E on glutathione content in red blood cells aqueous humor and lens of human and other species. Exp Eye Res 1986; 43: 905–914.
Martin Alonso J, Ghosh S, Coca Prados M . Cloning of bovine plasma selenium dependent glutathione peroxidase cDNA from the ocular ciliary epithelium. Expression of the plasma and cellular forms within the mammalian eye. Biochemistry 1993; 114: 284–291.
Quigley HA, West SK, Rodriguez J, Muñoz B, Klein R, Snyder R . The prevalence of glaucoma in a population-based study of hispanic subjects. Arch Ophthalmol 2001; 119: 1819–1826.
Foster PJ, Buhrmann R, Quigley HA, Johnson GJ . The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 2002; 86: 238–242.
Kyaw A . A simple colorimetric method for ascorbic acid determination in blood plasma. Clin Chim Acta 1978; 86: 153–157.
Lissi E, Pascual C, Del Castillo M . Luminol luminescence induced by 2,2-azo-bis (2-aminopropane) thermolysis. Free Rad Res Com 1992; 17: 299–311.
Flohé L, Gunzler WA . Assays of glutathione peroxidase. Methods Enzymol 1984; 105: 114–117.
Chance B, Sies H, Boveris A . Hydroperoxide metabolism in mammalian tissues. Physiol Rev 1979; 59: 527–605.
Misra HP, Fridovich L . The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972; 247: 3170–3175.
Koliakos G, Konstas A, Schlötzer-Scherardt U, Bufidis N, Ringvold A . Ascorbic acid concentration is reduced in the aqueous humor of patients with exfoliation syndrome. Am J Ophthalmol 2002; 134: 879–883.
Yimaz A, Adigüzel U, Tamer L, Yildirim O, Oz O, Vatansever H et al. Serum oxidant/antioxidant balance in exfoliation syndrome. Clin Exp Ophthalmol 2005; 33: 63–66.
Packer JE, Slater TF, Willson RL . Kinetic study of the reaction of vitamin C with vitamin E radicals (tocopheroxyls) in solutions. Nature 1979; 278: 737–738.
Zhou L, Higginbotham E, Yuc B . Effects of ascorbic on levels of fibronectin, laminin and collagen type1 in bovine trabecular meshwork in organ culture. Curr Eye Res 1998; 17: 211–217.
Kinsey V . Transfer of ascorbic acid and related compounds across the blood-aqueous barrier. Am J Ophthalmol 1947; 30: 1262–1266.
Bleich S, Roedl J, Von Ahsen N, Schlötzer-Scherardt U, Reulbach U, Beck G et al. Elevated homocysteine levels in aqueous humor of patients with pseudoexfoliation glaucoma. Am J Opthalmol 2004; 138: 162–164.
Bleich S, Junemann A, Von Ahsen N, Lausen B, Ritter K, Beck G et al. Homocysteine and risk of open-angle glaucoma. J Neural Transm 2002; 109: 1499–1504.
Vessani R, Ritch R, Liebmann J, Jofe M . Plasma homocysteine is elevated in patients with exfoliation syndrome. Am J Ophthalmol 2003; 136: 41–46.
Gartaganis SP, Georgakopoulos CD, Patsoukis NE, Gotsis S, Gartaganis V, Georgiou C . Glutathione and lipid peroxide changes in pseudoexfoliation syndrome. Curr Eye Res 2005; 30: 647–651.
Trachootham D, Lu W, Ogasawara M, Rivera-Del Valle N, Huang P . Redox regulation of cell survival. Antioxid Redox Signal 2008; 10: 1343–1375.
Brown G . Reversible binding and inhibition of catalase by nitric oxide. Eur J Biochem 1995; 232: 188–191.
Koliakos GG, Konstas A, Schlötzer-Schrehardt U, Hollo G, Mitova D, Kovatchev D et al. Endothelin-1 concentration is increased in aqueous humor of patients with exfoliation syndrome. Br J Ophthalmol 2004; 88: 523–527.
Izzotti A, Sacca SC, Cartiglia C, De Flora S . Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med 2003; 114: 638–646.
Izzotti A, Bagnis A, Sacca SC . The role of oxidative stress in glaucoma. Mutation Res 2006; 612: 105–114.
Kumar DM, Agarwal N . Oxidative stress en glaucoma: a burden of evidence. J Glaucoma 2007; 16: 334–343.
Aslan M, Cort A, Yucel I . Oxidative and nitrative stress markers in glaucoma. Free Rad Biol Med 2008; 45: 367–376.
Alvarado J, Murphy C, Polansky J, Juster R . Age related changes in trabecular meshwork cellularity. Invest Ophthalm Vis Sci 1981; 21: 714–727.
De la Paz M, Epstein D . Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest Ophthalm Vis Sci 1996; 37: 1849–1853.
Uçalhan O, Karel F, Kanpolat A, Deurime E, Durak I . Superoxide dismutase activity in the lens capsule of patients with pseudoexfoliation syndrome and cataract. J Cataract Refract Surg 2006; 32: 618–622.
Ophir A, Berenshtein E, Kitrossky N, Berman ER, Photiou S, Rothman Z et al. Hydroxyl radical generation in the cat retina during reperfusion following ischemia. Exp Eye Res 1993; 57: 351–357.
Acknowledgements
This study was supported by Grant B 124, University of Buenos Aires, Argentina and PIP 6320, CONICET, Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferreira, S., Lerner, S., Brunzini, R. et al. Antioxidant status in the aqueous humour of patients with glaucoma associated with exfoliation syndrome. Eye 23, 1691–1697 (2009). https://doi.org/10.1038/eye.2008.352
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2008.352
Keywords
This article is cited by
-
Evaluation of lacrimal drainage system in Pseudoexfoliation syndrome
Eye (2022)
-
Homeostatic alterations related to total antioxidant capacity, elemental concentrations and isotopic compositions in aqueous humor of glaucoma patients
Analytical and Bioanalytical Chemistry (2022)
-
Ascorbic acid concentrations in aqueous humor after systemic vitamin C supplementation in patients with cataract: pilot study
BMC Ophthalmology (2017)
-
Virus-mediated EpoR76E gene therapy preserves vision in a glaucoma model by modulating neuroinflammation and decreasing oxidative stress
Journal of Neuroinflammation (2016)
-
Influence of cataract maturity on aqueous humor lipid peroxidation markers and antioxidant enzymes
Eye (2014)