Abstract
Underlying cognitive declines in Alzheimer’s disease (AD) are the result of neuron and neuronal process losses due to a wide range of factors. To date, all efforts to develop therapies that target specific AD-related pathways have failed in late-stage human trials. As a result, an emerging consensus in the field is that treatment of AD patients with currently available drug candidates might come too late, likely as a result of significant neuronal loss in the brain. In this regard, cell-replacement therapies, such as human embryonic stem cell- or induced pluripotent stem cell-derived neural cells, hold potential for treating AD patients. With the advent of stem cell technologies and the ability to transform these cells into different types of central nervous system neurons and glial cells, some success in stem cell therapy has been reported in animal models of AD. However, many more steps remain before stem cell therapies will be clinically feasible for AD and related disorders in humans. In this review, we will discuss current research advances in AD pathogenesis and stem cell technologies; additionally, the potential challenges and strategies for using cell-based therapies for AD and related disorders will be discussed.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is clinically characterized by progressive loss of memory and other cognitive functions. Typically, several years pass between the initial onset of symptoms and eventual death. AD is estimated to have cost the US $172 billion and the world $604 billion in 2010 alone.1 These costs are staggering in light of predictions that the number of AD cases worldwide, currently estimated at 36 million, will triple by 2050.1 Therefore, there is a pressing need to identify novel mechanisms and develop new therapeutic strategies for AD. The complexity and multifactorial nature of AD poses unique challenges for pathogenic studies and therapeutic developments.2 Efforts to target AD-related pathways have shown promise in animal studies only to fail during human trials.2, 3 An emerging consensus in the field is that treatment of AD patients with currently available drug candidates comes too late, likely as a result of significant neuronal loss in the brain. In this regard, cell-replacement therapies, such as human embryonic stem cell (ESC)- or induced pluripotent stem cell (iPSC)-derived neural cells, hold potential for treating AD patients who may be beyond the help of pharmacological therapies.4 We will briefly review the current state of research in AD pathogenesis and new stem cell technologies. Additionally, the potential challenges and strategies for using cell-based therapies for AD and related disorders will be discussed. We will also highlight recent studies that have obtained or developed promising cell types that could be used to defeat this devastating disease in the future.
Advancement of research in AD pathogenesis
Genetics of AD pathogenesis
It is well known that the brains of AD patients accumulate two types of classically misfolded proteins. The first is amyloid-beta (Aβ), which is the pathological cleavage product of the amyloid precursor protein (APP).2 Aβ accumulates into plaques and smaller oligomers.2 Mutations in APP or in proteins involved in APP processing are well documented as being linked to inherited familial AD, an early-onset autosomal-dominant form of the disease that begins before the age of 65 years but only accounts for <2% of all AD cases.2 Many of the failed drugs in clinical trials directly or indirectly target this pathway with small molecules or antibody therapies to decrease Aβ production or promote Aβ clearance.2, 3 The second of the misfolded proteins in AD is tau, a microtubule-associated protein that accumulates intracellularly as neurofibrillary tangles, a pathological feature that most closely correlates with cognitive decline in AD.2 However, mutations in tau usually cause frontotemporal dementia but not AD.2
The vast majority (>98%) of AD cases, which do not involve mutations in genes of APP-processing pathways, are sporadic with onset beginning over the age of 65 years.2 For this population, the strongest predictor of developing AD, aside from age, is the genetic risk factor apolipoprotein (apo) E4.2 Each individual carries two copies of the apoE gene that exists in three allelic forms, ɛ2, ɛ3 and ɛ4, that encode three corresponding isoforms: apoE2, apoE3 and apoE4, respectively.5 Importantly, apoE4 carriers make up 60–75% of AD cases although those individuals only represent approximately 25% of the normal population. AD patients with apoE4 have a younger age of disease onset relative to non-carrier patients.6 All well-conducted genome-wide association studies on late-onset AD from different populations around the world have identified, with extremely high confidence, apoE4 as the top late-onset AD gene.7 Remarkably, the lifetime risk estimate of developing AD for individuals with two copies of the apoE4 allele (approximately 2% of the population) is approximately 60% by the age of 85 years and for those with one copy of the apoE4 allele (approximately 25% of the population), approximately 30%.8 In comparison, the lifetime risk of AD for those with two copies of the apoE3 allele is approximately 10% by the age of 85 years. Thus apoE4 should be considered a major gene with semi-dominant inheritance for late-onset AD.8 Interestingly, carriers of apoE2, the rarest isoform, have a decreased risk for developing AD compared with homozygous carriers of apoE3.6 Genome-wide association studies also identified other genes that modulate the risk of late-onset AD, including CLU, CR1, PICALM, BIN1, SORL1, GAB2, ABCA7, MS4A4/MS4A6E, CD2AP, CD33, EPHA1 and HLA-DRB1/5.7 However, the relative contribution of these genes to AD is modest compared with apoE4.
Aβ and AD pathogenesis
Diverse lines of evidence suggest that APP and Aβ contribute causally to the pathogenesis of early-onset familial AD, although to what extent they also contribute to late-onset sporadic AD is still unclear. Overexpression of APP in humans through duplication of its gene or trisomy of chromosome 21, which harbors the APP gene, causes early-onset AD, whereas partial trisomy 21 excluding the APP gene does not.9 The catalytic subunit of the γ-secretase protein complex, involved in releasing Aβ peptides from its precursor, is formed by presenilin 1 (PS1) or PS2. Autosomal-dominant mutations in APP, PS1 or PS2 that alter APP processing and the production or self-aggregation of Aβ also cause early-onset AD.7 Neuronal expression of mutant human APP (hAPP), either alone or in combination with mutant PS1 in transgenic rodents, causes several AD-like alterations.7, 8, 9, 10, 11, 12, 13 Aβ also causes synaptic dysfunction and other neuronal impairments when added to acute brain slices or primary neuronal cultures.11 Biochemical and animal studies have suggested that insoluble Aβ fibrils found in amyloid plaques and monomeric Aβ are less pathogenic than soluble, nonfibrillar assemblies of Aβ, such as Aβ dimers, trimers and larger oligomers.14 How exactly the different Aβ assemblies cause synaptic and neuronal dysfunction has been a topic of intense study and debate.11, 15 They may act inside or outside the cells and engage proteins as well as lipids.
ApoE4 and AD pathogenesis
Emerging evidence suggests that apoE4 has both Aβ-dependent and -independent roles in AD pathogenesis.2, 16, 17 In vivo, apoE is associated with amyloid plaques, and in vitro, it can form complexes with Aβ peptides.16, 17 Studies in apoE-deficient mice expressing mutant hAPP demonstrate that apoE is actually required for the formation of fibrillar amyloid plaques.18, 19 Interestingly, decreasing apoE’s lipidation status by knocking out ATP-binding cassette transporter A1 (ABCA1), a major regulator of cellular cholesterol and phospholipid homeostasis, in mutant hAPP mice significantly increases brain Aβ loads, whereas increasing apoE lipidation status by overexpressing ABCA1 decreases brain Aβ levels (for a review, see Kim et al.17). Thus, altering apoE lipidation changes its ability to mediate Aβ clearance or deposition in the brain. Furthermore, in hAPP transgenic mice, human apoE stimulates Aβ clearance, and apoE2 and apoE3 clear more Aβ than apoE4,18, 19 which may be related to apoE isoform-dependent effects on astroglial degradation of Aβ deposits.20 Microdialysis measurements of Aβ clearance rates in the brains of mutant hAPP transgenic mice expressing apoE3 or apoE4 reveal that apoE4 decreases Aβ clearance by approximately 40% compared with apoE3.21 Although apoE4 clearly increases Aβ accumulation and amyloid plaque formation in both humans and transgenic mouse models, it is still uncertain whether this process actually contributes to cognitive deficits in AD. As reported, plaque loads determined histopathologically or radiologically do not correlate well with cognitive impairments in humans.22 Furthermore, in the very oldest population (>90 years of age), the presence of apoE2 is associated with a reduced risk of dementia but an increased amyloid burden relative to apoE3.23
Both Aβ and apoE4 cause inhibitory interneuron impairments, contributing to learning and memory deficits
The GABAergic system is important in shaping learning and memory, especially in the hippocampus, a critical structure for the encoding of new episodic memories and spatial learning and navigation.24, 25 The dentate gyrus (DG), a subregion of the hippocampus, functions as a signaling gatekeeper between the entorhinal cortex and hippocampus in the processing of learning and memory tasks.24, 25 Learning triggers rapid increases in inhibitory synaptogenesis and gamma-amino-butyric acid (GABA) content at inhibitory synapses,26 which accompanies enhanced synaptic inhibition of excitatory neurons.27 Spatial learning also triggers a lasting increase in GABA release from hippocampal GABAergic interneurons.28, 29 Genetically enhancing GABAergic innervation in the DG of the hippocampus or increasing GABA levels by knocking down GABA transporter 1 improves learning and memory,30, 31 whereas decreasing GABA levels by overexpressing GABA transporter 1 is detrimental.32 Furthermore, optogenetically inhibiting the activity of even a small population of GABAergic interneurons in the DG of the hippocampus impairs learning and memory.33
Several lines of evidence suggest that Aβ regulates neuronal and synaptic activities and that accumulation of Aβ in the brain causes an intriguing combination of abnormally elevated network activity and synaptic depression.11 Impairment of inhibitory interneurons and aberrant stimulation of glutamate receptors, which can result in excitotoxicity, appear to have important upstream roles in this pathogenic cascade.2, 11, 34, 35, 36 Excessive neuronal activity might trigger a vicious positive feedback cycle by augmenting Aβ production, which is regulated, at least in part, by neuronal activity. This further destabilizes the network.37
ApoE4 impairs GABAergic inhibitory interneurons, contributing to AD pathogenesis.2 ApoE4 knock-in (KI) mice show an accelerated age-dependent decrease in hilar GABAergic interneurons, which correlates with the extent of apoE4-induced impairments of adult hippocampal neurogenesis and with learning and memory deficits.38, 39, 40 Interestingly, the detrimental effect of apoE4 on GABAergic interneurons is cell autonomous,41 which is important for potential stem cell transplantation therapy in AD patients with apoE4 (see below). In transgenic mice expressing neurotoxic apoE4 fragments, the loss of hilar interneurons is more pronounced and also correlates with learning and memory deficits.38 Tau removal prevents these adverse effects but not when GABA signaling is blocked with a low dose of picrotoxin.38 These findings strongly suggest that apoE4 causes age- and tau-dependent impairment of hilar GABAergic interneurons, leading to decreased neurogenesis in the hippocampus and learning and memory deficits. Recently, it has been reported that age-dependent hilar GABAergic interneuron impairment also correlates with learning and memory deficits in aged wild-type rats and mice.42, 43
Dysfunction of the GABAergic system may also contribute to cognitive impairment in humans. AD patients have decreased GABA and somatostatin (SST) levels in the brain and cerebral spinal fluid44, 45, 46, 47, 48 and these alterations are more severe in apoE4 carriers.49 ApoE4 is associated with increased brain activity during rest and in response to memory tasks,50, 51 possibly reflecting impaired GABAergic inhibitory control. With functional magnetic resonance imaging activation paradigms, mild cognitive impairment patients demonstrate hyperactivity in the medial temporal lobe,52, 53, 54, 55 and high-resolution functional magnetic resonance imaging indicates that hippocampal hyperactivity in mild cognitive impairment localizes to the DG/CA3 region of the hippocampus,56 paralleling findings in mice with apoE4-induced GABAergic hypofunction in the DG.38, 39, 40, 41 Treatment of apoE4-KI mice with the GABAA receptor potentiator pentobarbital or transplantation of mouse inhibitory neuron progenitors restores normal hippocampal activity and learning and memory, while blocking GABAergic signaling promotes the damaging effects of apoE4.38, 57 Likewise, reducing hippocampal hyperactivity with the antiepileptic levetiracetam improved cognition in patients with amnestic mild cognitive impairment and in a mouse model of AD.58, 59 These studies support the hypothesis that apoE4 contributes to AD pathogenesis, at least partially, by causing and exacerbating age-dependent impairment of GABAergic interneurons, leading to learning and memory deficits.2
Stem cell-based therapies in animal models of AD
As previously mentioned, multiple factors are involved in the pathogenesis of AD; these factors have not been successfully targeted by pharmaceutical or immunological agents.2 With the advancement of stem cell technologies and the ability to generate different types of neuronal and glial cells from stem cells, there is hope for stem cell therapeutics as novel treatments for AD. Toward this goal, some success with stem cell therapies has been made in various animal models of AD as a proof-of-concept.60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72
Neural stem cell (NSC)-based therapies in animal models of AD
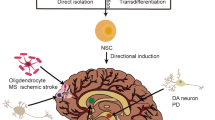
The ability of multipotent NSCs to differentiate into a variety of cell types, such as neurons, astrocytes and oligodendrocytes, at transplantation sites is especially promising for cell-replacement therapy. NSCs can be derived from primary tissues, including fetal, postmortem neonatal or adult brain tissues,72 or from ESCs and iPSCs.4, 73, 74
In mouse models of AD, studies have shown that transplanted mouse NSCs differentiate into mature cell types within the brain and improve learning and memory.75, 76 One study shows improvement of cholinergic neuron number and memory in fimbria-fornix-transected AD rats after transplantation with rat NSCs.77, 78 However, it is not clear whether this is due to differentiation, maturation and integration of the transplanted NSCs or whether their secreted factors and signaling molecules stimulate cholinergic neurogenesis and/or prevent further loss.70 Indeed, it has been shown that NSC grafts increase brain-derived neutrotrophic factor levels and lead to behavioral rescue without changing Aβ or tau pathologies in a mutant hAPP-overexpressing mouse model of AD.79 It seems that secretion of brain-derived neutrotrophic factor from the transplanted NSCs is required for rescuing cognitive function in AD transgenic mice, because shRNA-mediated brain-derived neutrotrophic factor knockdown abolishes the rescue.63, 79
Grafted NSCs can also be significantly influenced in their migration and differentiation by the microenvironment in recipient brains. For example, overexpression of hAPP causes grafts to yield more astrocytes rather than neurons.80 Thus the pathogenic process of AD may have a negative impact on the therapeutic effect of NSC transplantation. On the contrary, nerve growth factors are thought to promote survival and differentiation of transplanted NSCs. NSCs stably transduced with human nerve growth factor genes survive and integrate into the cerebral cortex of AD rats upon transplantation and enhance cognitive performance; this survival and integration is not observed in the same rat model transplanted with NSCs without genetic modification.64, 81
Transplantation of NSCs is also used as a vehicle to deliver potential therapeutic agents, including neprilysin, insulin-degrading enzyme, plasmin and cathepsin B, to decrease Aβ levels in AD mouse models.70 It has been reported that fibroblast-delivered neprilysin reduces amyloid plaques in AD mice.61, 82 Interestingly, delivery of the same gene by grafted NSCs leads to a more efficient reduction of amyloid plaques in mice. Thus it is suggested that future NSC-based therapy in AD should focus on such indirect mechanisms, in lieu of primary neuronal replacement, for the delivery of neurotrophic factors.62, 63, 71, 72, 74
GABAergic interneuron precursor-based therapies in animal models of AD
Cortical GABAergic interneurons are primarily produced in the embryonic medial ganglionic eminence (MGE).83, 84 The MGE is a transient embryonic structure in the ventral telencephalon from which immature progenitors of cortical interneurons originate, migrate and distribute throughout the cortex and hippocampus via tangential migration into the radially developing brain.85, 86 A number of studies demonstrate that this structure can be micro-dissected from developing rodent embryos and heterochronically transplanted into postnatal and adult animals. In these recipients, the transplanted MGE-derived interneuron progenitors migrate and integrate throughout recipient brains to alter ocular dominance plasticity87 or rescue models of stroke,88 anxiety,89 schizophrenia,90, 91 Parkinson’s disease92 or epilepsy,93, 94, 95, 96 which have been reviewed elsewhere.97, 98
Inhibitory interneuron impairments are a feature of both AD-related mouse models and human AD patients, and interneuron deficits seem to be a convergence point for apoE4 and Aβ mechanisms of the disease.2, 11 To determine whether replacing lost cells could restore neuronal network function and behavior, we transplanted embryonic MGE-derived interneuron progenitors into the hippocampal hilus of aged apoE4-KI mice with or without Aβ accumulation.57 Despite the toxic environment created by apoE4 alone or in combination with Aβ, in both conditions, the transplanted cells developed into mature interneurons, functionally integrated into the hippocampal circuitry and rescued learning and memory. Because the progenitor cells, which expressed wild-type mouse apoE, survived and integrated equally well into apoE3-KI and apoE4-KI mice (including those with significant Aβ plaque buildup), we provide further support for the model that the detrimental effects of apoE4 are cell autonomous. This is important for potential stem cell-based therapy of AD in the future, indicating that transplanted human MGE-like cells without apoE4 expression or Aβ overproduction would have a good chance to survive and functionally integrate in the brains of AD patients.
These studies demonstrate that MGE cells possess attractive characteristics for possible cell-based therapeutics: high capacity of migration, autonomous integration, subtype inhibitory differentiation, and circuit-modulation. A key aspect of GABAergic interneurons is that one such inhibitory neuron could connect to, and thus influence, more than a thousand excitatory neurons.99, 100 This suggests that the survival and functional integration of even small numbers of transplanted MGE cells could significantly improve learning and memory.
Derivation and transplantation of GABAergic inhibitory neuron precursors from PSCs
Murine MGE allograft transplantation studies are encouraging as proof-of-concept, but one of the ultimate goals for stem cell research is to develop human cell therapies. Correspondingly, one of the next steps for clinical translation would be to develop a reliable source of human MGE-like cells, particularly from PSCs, which could provide a potentially unlimited source of MGE cells for transplantation therapies of AD. Various protocols exist for the differentiation of mouse PSCs into cortical interneuron precursors.101, 102 In one study, a mouse PSC line with an Lhx6-GFP reporter was differentiated into cells expressing both FoxG1 and NKX2.1 using a modified protocol for the generation of ventral telencephalic cells.102 By day 12 of differentiation, many of these cells express Lhx6 and possess a differentiation pattern similar to MGE-derived progenitors upon transplantation. Interestingly, the study shows a bias in differentiation of the transplanted MGE-like cells towards SST+ interneurons upon maturation, which is attributed to higher levels of sonic hedgehog (SHH) signaling. Following this study, an enhanced protocol was developed for the generation of mouse PSC-derived cortical inhibitory neurons by the forced expression of NKX2.1 that could eliminate the need for sustained SHH expression.103 Mouse PSC-derived cortical inhibitory neurons were shown to be able to replace those neurons lost due to pilocarpine administration; indeed, mice that received these MGE-like inhibitory progenitors doubled the density of GABAergic interneurons in the hilus relative to control animals.104 Taken together, these and other studies105 provide substantial evidence that PSCs can serve as a renewable source of cortical interneuron progenitors.
Differentiation protocols for human PSCs have also proved encouraging, as several groups report the derivation of cortical interneuron progenitors from both human ESCs (hESC) and iPSCs.106, 107, 108, 109 Consistent with studies performed in mouse ESCs (mESCs), SHH signaling is also necessary for efficient patterning into MGE-like progenitors. A highly efficient method for generating MGE-like progenitors from hESCs (up to 93% NKX2.1+ without cell sorting) was developed, which relies on high concentrations of SHH.106 Upon transplantation, these human MGE-like cells mature into GABAergic interneurons as well as basal forebrain cholinergic neurons; in a mouse model depleted of these neuronal subtypes in the medial septum, the human cells restored short-term behavioral learning and memory deficits.106 Of note, the group reported no tumor formation in genetically immunodeficient mice transplanted with the hESC-derived MGE-like progenitors, likely due to the high purity of the differentiated cells and the absence of residual undifferentiated hESCs.106
Some studies on the efficient production of human inhibitory forebrain neurons utilize strategies, such as an intermediate MGE-progenitor state107 or a small-molecule-based strategy for the direct generation of forebrain inhibitory neurons.108 Nicholas et al.107 induced the differentiation of MGE-like progenitors from both ESCs and iPSCs into GABAergic interneurons with mature physiological properties, while Maroof et al.108 demonstrated the importance of SHH signaling for proper acquisition of the forebrain identity. Both studies display the ability of the transplanted PSC-derived neurons to survive, disperse from the injection site and integrate into mouse brains. Nicholas et al.107 also reports the absence of tumors from ESC-derived MGE cells in mice up to 7 months posttransplantation. Both studies emphasize an important facet of human development that is often difficult to mimic in in vitro studies: a protracted timeline of neuronal maturation.110 Indeed, both studies found the neurons generated to be immature with no fast spiking interneurons or with limited integration upon transplantation.107, 108 Further studies may be required to accelerate the generation of fully mature human GABAergic interneurons in order for these cells to be used in future AD therapies.
Conclusions, challenges, and perspectives
As discussed, cell-replacement therapies hold great potential for treating AD patients. With the advent of stem cell technologies and the ability to turn stem cells into different types of CNS neurons and glial cells, some success in stem cell therapy has been made in animal models of AD. Although these preclinical studies are promising, many more steps remain before stem cell therapies can be successfully used for the treatment of AD and related disorders.
NSCs and MGE-like inhibitory progenitors as candidates for stem cell-based therapies in AD
Requirements for neuronal-replacement therapy would entail the distribution of cells throughout the affected tissue by migration from the injection site while maintaining their intended identity, functional integration into or modulation of the crumbling circuitry and resistance to the same environmental toxins (misfolded or aggregated proteins) that cause the primary degenerative pathologies. For many neurodegenerative diseases, especially AD, multiple pathogenic factors and multiple neuronal systems are usually affected simultaneously.2 Thus a purely homogeneous source of neurons would need to be able to influence and/or protect a wide variety of other cell types and networks. This makes interneurons, and possibly NSCs, ideal candidates. Furthermore, NSC- and interneuron-based strategies are not mutually exclusive. It would be conceivable that interneurons could also be genetically engineered pretransplantation to deliver and secrete the neurotrophic factors that have shown some promise in NSC transplantation. These cells would theoretically retain their unique migratory capabilities and their ability to integrate and modulate the host network. Conversely, NSCs could be engineered to secrete GABA or inhibitory signaling potentiators to support inhibitory function of the brain networks.
Recently, a number of methods for generating induced NSCs (iNSCs) directly from fibroblasts have been reported.111, 112, 113, 114, 115, 116 iNSCs can also be derived from human astrocytes117 and sertoli cells.118 However, only some of these studies demonstrate viability and differentiation in vivo posttransplantation,111, 112, 113, 114 and none show any substantial rescue of behavior in mice upon transplantation. Most recently, though, it has been demonstrated that iNSCs can survive at least 6 months posttransplantation.119 Because this approach can generate patient-specific iNSCs for potential cell-replacement therapies in AD and related disorders, it is worthy of further study and improvement.
Progenitors with proliferation capability versus mature cells for transplantation
Other cellular features of donor cells, such as mitotic capacity and permanence of cell characteristics, and commitment to cell fate, should also be considered for any therapeutic cell type. For some strategies, it may be ideal for immature cells to divide a few times before structurally and functionally integrating into the circuitry. This requires fewer cells to be transplanted and is not as demanding on the cell source or immediate volume that the recipient tissue needs to accommodate; however, there is a legitimate concern about detrimental overgrowth in the form of tumors.62 Although it could be advantageous for cells to differentiate into multiple cell types or subtypes, randomly differentiating transplanted cells could introduce variability among patients and could prove deleterious in some cases.60, 120, 121
Variability of donor cells in stem cell-based therapies
Although hESCs and iPSCs have provided researchers with powerful tools for testing cell-replacement therapies, there is still much to be learned about their unique properties and culture conditions. Variability among differently established ESC lines has long been reported.122, 123 Because of non-standardized reprogramming methods and donor-to-donor variation, iPSCs in particular can also vary in their differentiation efficiencies and genetic backgrounds, which can affect downstream applications both for drug testing and transplantation. Comparisons of disease cases have often involved derivations of large numbers of patient-specific iPSC lines, which can be technically challenging and labor intensive. Thus more robust and efficient methods are needed to consistently derive a desired cell type, and each of those cell types needs more thorough characterization.
Donor cell and patient compatibility and immune rejection of stem cell-based therapies
Although the brain is considered to be ‘immune privileged,’ donor cells will have to be human leukocyte antigen haplotype-matched at the very least, and recipients would require some level of immunosuppression to prevent rejection of the transplanted cells. Ideally, there may exist the possibility of having patient-specific, isogenic genome-modified iPSC- or iNSC-derived cells when more reliable and efficient protocols are developed. Interestingly, in a transplant case of fetal midbrain dopaminergic cells that survived in a Parkinson’s disease patient for over 14 years, only a 6-month-long daily regimen of cyclosporin A was sufficient for prolonged survival.124, 125 Nevertheless, approaches to enhancing donor cell and patient compatibility and of suppressing immune rejection of transplanted cells are needed for future stem cell-based therapies in AD.
Regulatory approval and path to clinical use
Eventually, good manufacturing practices will need to be applied while handling all stages of transplantable cells for clinical use. Before making steps toward the clinic, if able to be manipulated in vitro, all grafted cells would ideally be transgenically equipped with a molecular ‘kill switch’ that could be easily activated in the event of adverse effects. Because AD can be a relatively slow-progressing disease, clinical trials will likely take many years to demonstrate success for cell therapies in halting or reversing disease progression. The safe and ethical future of stem cell therapies, especially for AD, will likely be slow, expensive and tightly controlled.62 However, due to the uniqueness of stem cell-based therapies, regulatory agents are needed to develop new regulatory policies to foster their appropriate development and success.
References
Wimo A, Prince M . World Alzheimer Report 2010. The Global Economic Impact of Dementia. London, England, 2010.
Huang Y, Mucke L . Alzheimer mechanisms and therapeutic strategies. Cell 2012; 148: 1204–1222.
Golde TE, Schneider LS, Koo EH . Anti-Aβ therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron 2011; 69: 203–213.
Yu DX, Marchetto MC, Gage FH . Therapeutic translation of iPSCs for treating neurological disease. Cell Stem Cell 2013; 12: 678–688.
Mahley RW, Apolipoprotein E . Cholesterol transport protein with expanding role in cell biology. Science 1988; 240: 622–630.
Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeus R et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. J Am Med Assoc 1997; 278: 1349–1356.
Bertram L, Lill CM, Tanzi RE . The genetics of Alzheimer disease: back to the future. Neuron 2010; 68: 270–281.
Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry 2011; 16: 903–907.
Prasher VP, Farrer MJ, Kessling AM, Fisher EMC, West RJ, Barber PC et al. Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann Neurol 1998; 43: 380–383.
Ashe KH, Zahs KR . Probing the biology of Alzheimer's disease in mice. Neuron 2010; 66: 631–645.
Palop JJ, Mucke L . Amyloid-β-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci 2010; 13: 812–818.
Marchetti C, Marie H . Hippocampal synaptic plasticity in Alzheimer's disease: What have we learned so far from transgenic models? Rev Neurosci 2011; 22: 373–402.
Querfurth HW, LaFerla FM . Alzheimer's disease. N Engl J Med 2010; 362: 329–344.
Mucke L, Selkoe DJ . Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. In: Selkoe DJ, Mandelkow E, Holtzman DM (eds). The Biology of Alzheimer Disease. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2011, pp 317–333.
Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ . Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci 2011; 31: 6627–6638.
Huang Y . Apolipoprotein E and Alzheimer disease. Neurology 2006; 66 (Suppl. 1): S79–S85.
Kim J, Basak JM, Holtzman DM . The role of apolipoprotein E in Alzheimer's disease. Neuron 2009; 63: 287–303.
Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 2000; 97: 2892–2897.
Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J et al. Apolipoprotein E is essential for amyloid deposition in the APPV717F transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 1999; 96: 15233–15238.
Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat Med 2004; 10: 719–726.
Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med 2011; 3: 89ra57.
Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology 2003; 60: 1495–1500.
Berlau DJ, Corrada MM, Head E, Kawas CH . APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology 2009; 72: 829–834.
Squire LR, Wixted JT . The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci 2011; 34: 259–288.
Wang SH, Morris RGM . Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 2010; 61: 49–79.
Jasinska M, Siucinska E, Cybulska-Klosowicz A, Pyza E, Furness DN, Kossut M et al. Rapid, learning-induced inhibitory synaptogenesis in murin barrel field. J Neurosci 2010; 30: 1176–1184.
Brosh I, Barkai E . Learning-induced enhancement of feedback inhibitory synaptic transmission. Learn Mem 2009; 16: 413–416.
Nitz D, McNaughton B . Differential modulation of CA1 and dentate gyrus interneurons during exploration of novel environments. J Neurophysiol 2004; 91: 863–872.
Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 2008; 135: 549–560.
Morellini F, Sivukhina E, Stoenica L, Oullanova E, Bukalo O, Jakovcevski I et al. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb Cortex 2010; 20: 2712–2727.
Shi J, Cai Y, Liu G, Gong N, Liu Z, Xu T et al. Enhanced learning and memory in GAT1 heterozygous mice. Acta Biochem Biophys Sin 2012; 44: 359–366.
Hu JH, Ma YH, Jiang J, Yang N, Duan SH, Jiang ZH et al. Cognitive impairment in mice over-expressing gamma-aminobutyric acid transporter 1 (GAT1). Neuroreport 2004; 15: 9–12.
Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I et al. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PLoS ONE 2012; 7: e40555.
Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 2012; 149: 708–721.
Meilandt WJ, Yu GQ, Chin J, Roberson ED, Palop JJ, Wu T et al. Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci 2008; 28: 5007–5017.
Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou Y, Halabisky B et al. Phospholipase A2 reduction ameliorates cognitve deficits in mouse model of Alzheimer’s disease. Nat Neurosci 2008; 11: 1311–1318.
Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichie ME et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci 2011; 14: 750–756.
Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci 2010; 30: 13707–13717.
Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K et al. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell 2009; 5: 634–645.
Leung L, Andrews-Zwilling Y, Yoon SY, Ring K, Jain S, Walker D et al. Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice. PLoS ONE 2012; 7: e53569.
Knoferle J, Yoon SY, Walker D, Leung L, Gillespie A, Tong L et al. Apolipoprotein E4 produced in GABAergic interneurons causes learning and memory deficits in mice. J Neurosci 2014; 34: 14069–14078.
Spiegel AM, Koh MT, Vogt NM, Rapp PR, Gallagher M . Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol 2013; 521: 3508–3523.
Koh MT, Spiegel AM, Gallagher M . Age-associated changes in hippocampal-dependent cognition in diversity outbred mice. Hippocampus 2014; 24: 1300–1307.
Bareggi SR, Franceschi M, Bonini L, Zecca L, Smirne S . Decreased CSF concentrations of homovanillic acid and γ-aminobutyric acid in Alzheimer's disease. Age- or disease-related modifications? Arch Neurol 1982; 39: 709–712.
Davies P, Katzman R, Terry RD . Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementia. Nature 1980; 288: 279–280.
Hardy J, Cowburn R, Barton A, Reynolds G, Dodd P, O'Carroll AM et al. A disorder of cortical GABAergic innervation in Alzheimer's disease. Neurosci Lett 1987; 73: 192–196.
Seidl R, Cairns N, Singewald N, Kaehler ST, Lubec G . Differences between GABA levels in Alzheimer's disease and Down syndrome with Alzheimer-like neuropathology. Naunyn Schmiedeberg Arch Pharmacol 2001; 363: 139–145.
Zimmer R, Teelken AW, Trieling WB, Weber W, Weihmayr T, Lauter H . γ-Aminobutyric acid and homovanillic acid concentration in the CSF of patients with senile dementia of Alzheimer's type. Arch Neurol 1984; 41: 602–604.
Grouselle D, Winsky-Sommerer R, David JP, Delacourte A, Dournaud P, Epelbaum J . Loss of somatostatin-like immunoreactivity in the frontal cortex of Alzheimer patients carrying the apolipoprotein epsilon 4 allele. Neurosci Lett 1998; 255: 21–24.
Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA et al. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement 2010; 6: 303–311.
Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA 2009; 106: 7209–7214.
Dickerson BC, Salat DH, Bales JF, Atiya M, Killany RJ, Greve DN et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol 2004; 56: 27–35.
Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 2005; 65: 404–411.
Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci 2006; 26: 10222–10231.
Hämäläinen A, Pihaljamäki M, Tanila H, Hänninen T, Niskanen E, Tervo S et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging 2007; 28: 1889–1903.
Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE . High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage 2010; 51: 1242–1252.
Tong LM, Djukic B, Arnold C, Gillespie AK, Yoon SY, Wang MM et al. Inhibitory interneuron progenitor transplantation restores normal learning and memory in apoE4 knock-in mice without or with Aβ accumulation. J Neurosci 2014; 34: 9506–9515.
Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 2012; 74: 467–474.
Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc Natl Acad Sci USA 2012; 109: E2895–E2903.
Andressen C . Neural stem cells: from neurobiology to clinical applications. Curr Pharm Biotechnol 2013; 14: 20–28.
Choi SS, Lee SR, Kim SU, Lee HJ . Alzheimer’s disease and stem cell therapy. Exp Neurobiol 2014; 23: 45–52.
Dunnett SB, Rosser AE . Challenges for taking primary and stem cells into clinical neurotransplantation trials for neurodegenerative disease. Neurobiol Dis 2014; 61: 79–89.
Chen WW, Blurton-Jones M . Concise review: can stem cells be used to treat or model Alzheimer’s disease? Stem Cells 2012; 30: 2612–2618.
Chen C, Xiao SF . Induced pluripotent stem cells and neurodegenerative diseases. Neurosci Bull 2011; 27: 107–114.
Fan X, Sun D, Tang X, Cai Y, Yin ZQ, Xu H . Stem-cell challenges in the treatment of Alzheimer’s disease: a long way from bench to bedside. Med Res Rev 2014; 34: 957–978.
Glat MJ, Offen D . Cell and gene therapy in Alzheimer’s disease. Stem Cells Dev 2013; 22: 1490–1496.
Kim SU, de Vellis J . Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res 2009; 87: 2183–2200.
Li M, Guo K, Ikehara S . Stem cell treatment for Alzheimer's disease. Int J Mol Sci 2014; 15: 19226–19238.
Borlongan CV . Recent preclinical evidence advancing cell therapy for Alzheimer's disease. Expt Neurol 2012; 237: 142–146.
Kim SU, Lee HJ, Kim YB . Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology 2013; 33: 491–504.
Liu AKL . Stem cell therapy for Alzheimer’s disease: hype or hope? Biosci Horizons 2013; 6: hzt011.
Martínez-Morales PL, Revilla A, Ocana I, Gonzalez C, Sainz P, McGuire D et al. Progress in stem cell therapy for major human neurological disorders. Stem Cell Rev 2013; 9: 685–699.
Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE 2011; 6: e17540.
Hermann A, Storch A . Induced neural stem cells (iNSCs) in neurodegenerative diseases. J Neural Transm 2013; 120 (Suppl): S19–S25.
Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 2007; 25: 1204–1212.
Yamasaki TR, Blurton-Jones M, Morrissette DA, Kitazawa M, Oddo S, LaFerla FM . Neural stem cells improve memory in an inducible mouse model of neuronal loss. J Neurosci 2007; 27: 11925–11933.
Xuan AG, Luo M, Ji WD, Long DH . Effects of engrafted neural stem cells in Alzheimer’s disease rats. Neurosci Lett 2009; 450: 167–171.
Xuan AG, Long DH, Gu HG, Yang DD, Hong LP, Leng SL . BDNF improves the effects of neural stem cells on the rat model of Alzheimer’s disease with unilateral lesion of fimbria-fornix. Neurosci Lett 2008; 440: 331–335.
Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA 2009; 106: 13594–13599.
Kwak YD, Brannen CL, Qu T, Kim HM, Dong X, Soba P et al. Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev 2006; 15: 381–389.
Wu S, Sasaki A, Yoshimoto R, Kawahara Y, Manabe T, Kataoka K et al. Neural stem cells improve learning and memory in rats with Alzheimer’s disease. Pathobiology 2008; 75: 186–194.
Chen SQ, Cai Q, Shen YY, Wang PJ, Teng GJ, Li MH et al. 1)H-MRS evaluation of therapeutic effect of neural stem cell transplantation on Alzheimer’s disease in AβPP/PS1 double transgenic mice. J Alzheimers Dis 2012; 28: 71–80.
Anderson S, Mione M, Yun K, Rubenstein JL . Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex 1999; 9: 646–654.
Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ . A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci 2011; 31: 10948–10970.
Marín O, Rubenstein JL . A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci 2001; 2: 780–790.
Marin O . Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development 2003; 130: 1889–1901.
Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP . Cortical plasticity induced by inhibitory neuron transplantation. Science 2010; 327: 1145–1148.
Daadi MM, Lee SH, Arac A, Grueter BA, Bhatnagar R, Maag AL et al. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant 2009; 18: 815–826.
Valente MF, Romariz S, Calcagnotto ME, Ruit L, Mello LE, Frussa-Filho R et al. Postnatal transplantation of interneuronal precursor cells decreases anxiety-like behavior in adult mice. Cell Transplant 2013; 22: 1237–1247.
Perez SM, Lodge DJ . Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Mol Psychiatry 2013; 18: 1193–1198.
Gilani AI, Chohan MO, Inan M, Schobel SA, Chaudhury NH, Paskewitz S et al. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc Natl Acad Sci USA 2014; 111: 7450–7455.
Martínez-Cerdeño V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell 2010; 6: 238–250.
Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci USA 2009; 106: 15472–15477.
Waldau B, Hattiangady B, Kuruba R, Shetty AK . Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells 2010; 28: 1153–1164.
Zipancic I, Calcagnotto ME, Piquer-Gil M, Mello LE, Alvarez-Dolado M . Transplant of GABAergic precursors restores hippocampal inhibitory function in a mouse model of seizure susceptibility. Cell Transplant 2010; 19: 549–564.
Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC . GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci 2013; 16: 692–697.
Tyson JA, Anderson SA . GABAergic interneuron transplants to study development and treat disease. Trends Neurosci 2014; 37: 169–177.
Calcagnotto ME, Zipancic I, Piquer-Gil M, Mello LE, Alvarez-Dolado M . Grafting of GABAergic precursors rescues deficits in hippocampal inhibition. Epilepsia 2010; 51 (Suppl 3): 66–70 (2010).
Morgan RJ, Santhakumar V, Soltesz I . Modeling the dentate gyrus. Prog Brain Res 2007; 163: 639–658.
Amaral DG, Scharfman HE, Lavenex P . The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 2007; 163: 3–22.
Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 2005; 8: 288–296.
Maroof AM, Brown K, Shi SH, Studer L, Anderson SA . Prospective isolation of cortical interneuron precursors from mouse embryonic stem cells. J Neurosci 2010; 30: 4667–4675.
Petros TJ, Maurer CW, Anderson SA . Enhanced derivation of mouse ESC-derived cortical interneurons by expression of Nkx2.1. Stem Cell Res 2013; 11: 647–656.
Maisano X, Litvina E, Tagliatela S, Aaron GB, Grabel LB, Naegele JR . Differentiation and functional incorporation of embryonic stem cell-derived GABAergic interneurons in the dentate gyrus of mice with temporal lobe epilepsy. J Neurosci 2012; 32: 46–61.
Goulburn AL, Stanley EG, Elefanty AG, Anderson SA . Generating GABAergic cerebral cortical interneurons from mouse and human embryonic stem cells. Stem Cell Res 2012; 8: 416–426.
Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L et al. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat Biotechnol 2013; 31: 1–10.
Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 2013; 12: 573–586.
Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 2013; 12: 559–572.
Kim TG, Yao R, Monnell T, Cho JH, Vasudevan A, Koh A et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. Stem Cells 2014; 32: 1789–1804.
Marín O . Human cortical interneurons take their time. Cell Stem Cell 2013; 12: 497–499.
Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M . Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci USA 2012; 109: 2527–2532.
Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 2012; 10: 465–472.
Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 2012; 10: 473–479.
Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwiling Y, Li G et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 2012; 11: 100–109.
Zhou Q, Tripathi P . How to remake a fibroblast into a neural stem cell. Cell Stem Cell 2012; 10: 347–348.
Kim SM, Flaβkamp H, Hermann A, Arauzo-Bravo MJ, Lee SC, Lee SH et al. Direct conversion of mouse fibroblasts into induced neural stem cells. Nat Protoc 2014; 9: 871–881.
Corti S, Nizzardo M, Simone C, Falcone M, Donadoni C, Salani S et al. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res 2012; 318: 1528–1541.
Sheng C, Zheng Q, Wu J, Xu Z, Wang L, Li W et al. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res 2012; 22: 208–218.
Hemmer K, Zhang M, van Wullen T, Sakalem M, Tapia N, Baumuratov A et al. Induced neural stem cells achieve long-term survival and functional integration in the adult mouse brain. Stem Cell Rep 2014; 3: 423–431.
Hitoshi S, Tropepe V, Ekker M, van der Kooy D . Neural stem cell lineages are regionally specified, but not committed, within distinct compartments of the developing brain. Development 2002; 244: 233–244.
Okano H, Temple S . Cell types to order: temporal specification of CNS stem cells. Curr Opin Neurobiol 2009; 19: 112–119.
Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 2011; 144: 439–452.
Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol 2011; 29: 279–286.
Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson’s disease. Brain 2005; 128: 1498–1510.
Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O . Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep 2014; 7: 1755–1761.
Acknowledgements
This work was supported in part by grants AG048030, NS079725, AG047655 and AG023501 from the National Institutes of Health, the SD Bechtel, Jr. Foundation, the Roddenberry Foundation and the Hellman Foundation. We thank Laura Leung and Philip Nova for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Tong, L., Fong, H. & Huang, Y. Stem cell therapy for Alzheimer’s disease and related disorders: current status and future perspectives. Exp Mol Med 47, e151 (2015). https://doi.org/10.1038/emm.2014.124
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2014.124
This article is cited by
-
piRNAs Interact with Cold-Shock Domain-Containing RNA Binding Proteins and Regulate Neuronal Gene Expression During Differentiation
Molecular Neurobiology (2022)
-
Stem cell-based therapy as a promising approach in Alzheimer's disease: current perspectives on novel treatment
Cell and Tissue Banking (2021)
-
In vitro differentiation of cGMP-grade retinal pigmented epithelium from human embryonic stem cells
International Journal of Retina and Vitreous (2019)
-
Melatonin-pretreated adipose-derived mesenchymal stem cells efficeintly improved learning, memory, and cognition in an animal model of Alzheimer's disease
Metabolic Brain Disease (2019)
-
An Integrated Miniature Bioprocessing for Personalized Human Induced Pluripotent Stem Cell Expansion and Differentiation into Neural Stem Cells
Scientific Reports (2017)