Abstract
Cytokines activate several inflammatory signals that mediate β-cell destruction. We recently determined that SPA0355 is a strong anti-inflammatory compound, thus reporting its efficacy in protecting β cells from various insults. The effects of SPA0355 on β-cell survival were studied in RINm5F cells and primary islets. The protective effects of this compound on the development of type 1 diabetes were evaluated in non-obese diabetic (NOD) mice. SPA0355 completely prevented cytokine-induced nitric oxide synthase (iNOS) expression and cytotoxicity in RINm5F cells and isolated islets. The molecular mechanism of SPA0355 inhibition of iNOS expression involves the inhibition of nuclear factor κB and Janus kinase signal transducer and activator of transcription pathways. The protective effects of SPA0355 against cytokine toxicity were further demonstrated by normal insulin secretion and absence of apoptosis of cytokine-treated islets. In experiments with NOD mice, the occurrence of diabetes was efficiently reduced when the mice were treated with SPA0355. Therefore, SPA0355 might be a valuable treatment option that delays the destruction of pancreatic β cells in type 1 diabetes.
Similar content being viewed by others
Introduction
Nitric oxide (NO), generated by the inducible NO synthase (iNOS), performs a wide variety of physiological and pathophysiological functions. Aberrant iNOS expression has a critical role in various inflammatory diseases such as rheumatoid arthritis,1 asthma,2 septic shock3 and inflammatory bowel disease.4 It is also known that the induction of iNOS and NO production is the essential step for the pathogenesis of type 1 diabetes.5, 6 Excess NO causes aberrant mitochondrial metabolism, protein modification and DNA cleavage, any one of which could lead to impaired insulin secretion and β-cell death.7 It has been proposed that the activation of the nuclear factor κB (NF-κB) pathway and/or the Janus kinase (JAK) signal transducer and activator of transcription (STAT) pathway is important for the induction of iNOS synthesis.8, 9 It can therefore be inferred that blockage of any of the NF-κB and JAK-STAT activation pathways would be useful in preventing the death or dysfunction of β cells.
We have recently reported that SPA0355 inhibits the inflammatory responses in an animal experimental model of arthritis by suppressing the NF-κB pathway.10 This suggests that SPA0355 may be effective for treating other types of NF-κB-related inflammatory diseases. We therefore examined the effects of this compound on the pathogenesis of type 1 diabetes. More specifically, we investigated the effects of SPA0355 on cytokine-induced NF-κB and JAK-STAT activation in cultured insulinoma cells and isolated islets. We further investigated whether type 1 diabetes was suppressed and whether the functional β-cell mass was preserved with intraperitoneal administration of SPA0355 in spontaneous autoimmune non-obese diabetic (NOD) mice.
Materials and methods
Cell culture and reagents
Rat pancreatic β-cell lines (RINm5F cells) were acquired from the American Type Culture Collection (Manassas, VA, USA). Interleukin-1β (IL-1β) and interferon-γ (IFN-γ) were obtained from R&D Systems (Minneapolis, MN, USA). All reagents were from Sigma-Aldrich (St Louis, MO, USA), unless otherwise noted.
Mice
Female NOD (H-2g7) and NOD/severe combined immunodeficient (SCID) mice were purchased from the Jackson Lab (Bar Harbor, ME, USA). The mice were housed in a laminar flow cabinet and maintained on standard laboratory chow ad libitum. All the animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 2011). This study protocol was approved by the Institutional Animal Care and Use Committee of Chonbuk National University.
Preparation of SPA0355
SPA0355 (1-methyl-3-[4-(2-phenoxazin-10-ylethoxy)phenyl]thiourea) was prepared as previously described.10
MTT assay for cell viability
The viability of cultured cells was determined using the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide to formazan. RINm5F cells were seeded overnight in clear, flat-bottomed 96-well tissue culture plates at 105 cells per well in 100 μl of medium. The cells were pre-treated with SPA0355 for 3 h, and IL-1β (1 U ml–1) and IFN-γ (100 U ml–1) were added for an additional 48 h. The cells were washed twice with phosphate-buffered saline and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide was added (100 μg/100 μl phosphate-buffered saline). After incubation at 37 °C for 1 h, 100 μl dimethylsulphoxide was added to dissolve the formazan crystals, and the absorbance was measured at 570 nm.
NO measurement
Biologically produced NO is rapidly oxidized to nitrite and nitrate in aqueous solutions. NO production was measured as nitrite concentration in cell-free culture supernatants using a colorimetric assay. Briefly, 5 × 106 RINm5F cells or 30 islets were pre-treated with the indicated concentrations of SPA0355 for 3 h before the addition of IL-1β (1 U ml–1) and IFN-γ (100 U ml–1). After 24 h, 100 μl aliquots of the culture supernatant were incubated at room temperature for 5 min with 100 μl of modified Griess reagent with a 1:1 mixture of 1% sulfanilamide in 30% acetic acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in 60% acetic acid, and the absorbance was measured at 540 nm. The NO concentration was determined using a linear standard curve of serial dilutions of sodium nitrite in a working medium.
Western blot analysis
Cells or islets were homogenized in 100 μl of ice-cold lysis buffer (20 mM Hepes [pH 7.2], 1% Triton X-100, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg ml–1 leupeptin and 10 μg ml–1 aprotinin), and 20 μg of protein was separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis before transfer to nitrocellulose membranes. The blots were probed with primary antibodies (1 μg ml–1) against p50, p65, IκBα, suppressor of cytokine signaling (SOCS)-1, SOCS-3, iNOS, proliferating cell nuclear antigen and β-actin (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) or IκB kinase-α (IKKα), IKKβ, p-IKKα, p-IKKβ, STAT-1, STAT-3, p-STAT-1 and p-STAT-3 (all from Cell Signaling, Beverly, MA, USA). Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology) was used as a secondary antibody.
RNA isolation and real-time reverse transcriptase-PCR
RNA was isolated from RINm5F cells or islets using Trizol (Invitrogen, Carlsbad, CA, USA), precipitated with isopropanol and dissolved in diethylpyrocarbonate-treated distilled water. Total RNA (2 μg) was treated with RNase-free DNase (Invitrogen), and first-strand complementary DNA was generated using a random hexamer primer with a first-strand complementary DNA synthesis kit (Applied Biosystems, Foster City, CA, USA). Specific primers for iNOS were designed using primer express software (Applied Biosystems): iNOS (accession no. NM_012611) forward, 5′-TGTGCTAATGCGGAAGGTCAT-3′, and iNOS reverse, 5′-CGACTTTCCTGTCTCAGTAGCAAA-3′. Control 18S ribosomal RNA was purchased from Applied Biosystems and used as the invariant control. The real-time reverse transcriptase-PCR mixtures consisted of 10 ng of reverse transcribed total RNA, 167 nmol l–1 forward and reverse primers, and 2 × PCR master mix in a final volume of 10 μl. The reactions were carried out in 384-well plates using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems).
Preparation of cytosolic and nuclear protein extracts
Cells or islets were washed with phosphate-buffered saline and lysed in CytoBuster Protein Extraction Buffer (Novagen, Madison, WI, USA). The lysate was centrifuged at 10 000 × g for 5 min at 4 °C, and the supernatant was used as the whole-cell protein extract. Cytoplasmic and nuclear extracts were prepared from cells using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL, USA).
Electrophoretic mobility shift assay
Nuclear extracts prepared from the cells or islets were incubated in a proteinase inhibitor cocktail (Calbiochem, San Diego, CA, USA) to inhibit endogenous protease activity. An oligonucleotide containing the κ-chain binding site (5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) was synthesized and used as a probe in a gel retardation assay. The two complementary strands were then annealed and labeled with α-32PdCTP. Labeled oligonucleotides (10 000 counts per minute), 10 μg of nuclear extracts and binding buffer (10 mM Tris–HCl [pH 7.6], 500 mM KCl, 10 mM EDTA, 50% glycerol, 100 ng poly[dI·dC] and 1 mM dithiothreitol) were then incubated for 30 min at room temperature in a final volume of 20 μl. Next, the reaction mixtures were analyzed by electrophoresis on 4% polyacrylamide gels in a 0.5 × Tris-borate buffer, and the gels were dried and examined using autoradiography. The specificity of the DNA–protein interaction for NF-κB was confirmed via competition assays using a 50-fold excess of unlabeled oligonucleotide.
Isolation of islets and determination of their viability
Pancreatic islets were isolated from male Sprague–Dawley rats (Orientbio, Seoul, Korea) using the collagenase digestion method. The viability of the islets was evaluated as described previously11 and was determined by hematoxylin and eosin staining and labeling of anti-insulin antibodies (Santa Cruz Biochemicals, Santa Cruz, CA, USA). Apoptosis was determined using the APOPercentage apoptosis assay kit (Biocolor Ltd., Belfast, Ireland).
Glucose-stimulated insulin secretion assay
Islets were cultured for 24 h with IL-1β (1 U ml–1) and IFN-γ (100 U ml–1) in the presence or absence of SPA0355 and subsequently washed three times in Krebs–Ringer bicarbonate buffer (25 mM Hepes [pH 7.4], 115 mmol l–1 NaCl, 24 mmol l–1 NaHCO3, 5 mmol l–1 KCl, 1 mmol l–1 MgCl2, 2.5 mmol l–1 CaCl2 and 0.1% bovine serum albumin) containing 2.8 mmol l–1 D-glucose. Insulin secretion assays were performed with 2.8 or 16.7 mmol l–1 D-glucose and measured using an ELISA kit (Millipore, Bedford, MA, USA).
Adoptive transfer experiments
Eight-week-old NOD/SCID female mice were divided into two groups (n=10 per group), and the mice were injected intraperitoneally at 2-day intervals with SPA0355 (1 mg kg–1) or corn oil for the entire experimental period. For disease transfer, 1 × 107 freshly isolated splenocytes from diabetic NOD mice were intravenously injected into NOD/SCID mice that were pre-treated with SPA0355 or vehicle for 6 days. Diabetes development was determined by measurement of blood glucose levels (over 300 mg dl–1). Blood glucose was assayed using a glucometer (Roche, Mannheim, Germany). To determine the effect of SPA0355 on macrophage activation, we performed separate experiment with three mice per group, and the mice were killed 10 days after adoptive transfer.
Flow cytometry
For cell surface protein staining, cells were removed from spleens and incubated with fluorescein isothiocyanate-conjugated anti-CD80 and allophycocyanin (APC) anti-F4/80 antibodies. Subsequently, the cells were washed and fixed with 4% formaldehyde for 20 min at room temperature and analyzed. For intracellular cytokine analysis of tumor necrosis factor-α, splenic cells (1 × 106 cells ml–1) were stimulated for 4 h with 10 ng ml–1 phorbol myristate acetate and ionomycin (1 μM) in the presence of brefeldin A (10 μg ml–1) to block cytokine secretion and facilitate intracellular accumulation. The antibodies used were fluorescein isothiocyanate-conjugated anti-tumor necrosis factor-α antibody and phycoerythrin-conjugated anti-CD4 antibody. Stained cells were evaluated by flow cytometry analysis using an Accuri flow cytometer (BD Biosciences, San Jose, CA, USA). All antibodies and recombinant cytokines were purchased from eBioscience (San Diego, CA, USA).
The induction of type 1 diabetes in NOD mice
Eight-week old female NOD mice were intraperitoneally injected with SPA0355 (1 mg kg–1) or corn oil at 2-day intervals. Diabetes development was determined by weekly measurement of blood glucose levels (>300 mg dl–1 was considered diabetic). Blood glucose was assayed using a glucometer (Roche). Surviving mice were killed at the age of 33 weeks, and pancreata were removed. For insulitis assessment, pancreata were fixed in 10% formalin and embedded in paraffin. Sections (7 μm thickness) were cut at 100 μm intervals to prevent counting the same islet twice. Two sections per pancreas were stained with hematoxylin and eosin and analyzed by light microscopy. Insulitis was scored according to the following criteria: ‘severe’ (⩾50% of the islet area infiltrated), ‘mild’ (<50% of the islet area infiltrated), ‘peri-insulitis’ (infiltration restricted to the islet periphery) and ‘no insulitis’ (absence of cell infiltration).
Immunohistochemistry
Immunohistochemical staining was performed with the DAKO Envision system (DAKO, Carpinteria, CA, USA), and dextran polymers conjugated with horseradish peroxidase were used to avoid contamination with endogenous biotin. Pancreata were removed and subsequently placed in fixative (10% formalin solution in 0.1 mol l–1 phosphate-buffered saline). Histological sections (4 μm) were cut from formalin-fixed, paraffin-embedded tissue blocks. After deparaffinization, the tissue sections were treated using a microwave antigen retrieval procedure in 0.01 mol l–1 sodium citrate buffer. The activity of endogenous peroxidase was blocked. Then, the sections were incubated with serum-free protein block (DAKO) to block nonspecific staining and subsequently incubated with anti-insulin antibodies (Santa Cruz Biochemicals). Peroxidase activity was detected with 3-amino-9-ethyl carbazole.
Statistical analysis
Statistical analysis was performed using analysis of variance and Duncan’s tests. A P-value of <0.05 was considered statistically significant.
Results
Effects of SPA0355 on cytokine-mediated cell death and iNOS expression in RINm5F cells
To determine the β-cell protective effects of SPA0355, we first examined the efficacy of this compound in protecting RINm5F cells from cytokine toxicity. The results showed that cell viability was significantly reduced to 53.4±5.9% compared with the control following treatment with cytokines (Figure 1a). The viability of cytokine-treated RINm5F cells was increased in a concentration-dependent manner following pre-treatment with SPA0355. However, the cell viability remained unchanged during treatment with SPA0355 alone.
SPA0355 prevents cytokine-induced cell death in RINm5F cells. RINm5F cells were pre-treated with the indicated concentrations of SPA0355 for 3 h, and interleukin-1β (IL-1β) and interferon-γ (IFN-γ) were added. (a) Following a 48-h incubation, cell viability was determined using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. (b) Following a 24-h incubation, the level of nitrite production and the degree of the expression of inducible nitric oxide synthase (iNOS) mRNA and protein were determined. Each value is the mean±s.e.m. of three independent experiments (n=9). **P<0.01 vs untreated control; #P<0.05, ##P<0.01 vs cytokine treatment.
NO production and iNOS expression were subsequently evaluated. At 24 h, nitrite was generated at concentrations of 6.9±0.3 μM and 20±0.3 μM from control and cytokine-treated RINm5F cells, respectively (Figure 1b). Moreover, the levels of iNOS mRNA and protein were also increased following treatment with cytokines. However, following pre-treatment with SPA0355, the degree of NO production and the levels of iNOS mRNA and protein were decreased in a concentration-dependent manner.
Effects of SPA0355 on cytokine-induced NF-κB activation in RINm5F cells
We examined the effects of SPA0355 on cytokine-stimulated NF-κB activation. In cytokine-treated RINm5F cells, there was an increase in the DNA-binding ability of the NF-κB subunit (Figure 2a) and a decrease in the protein level of inhibitory factor of NF-κB (IκB)-α in the cytosol (Figure 2b), both of which were markedly suppressed following treatment with SPA0355. We also examined the effects of SPA0355 on IKK activation, which is an essential process for the phosphorylation and degradation of IκB. The results showed that SPA0355 had no effect on the levels of IKKα and IKKβ; however, it suppressed the cytokine-induced phosphorylation of IKKα and IKKβ (Figure 2b). These results suggest that SPA0355 inhibits NF-κB activation by reducing IKK activities, thus preventing iNOS expression.
SPA0355 inhibits cytokine-induced nuclear factor κB (NF-κB) activation in RINm5F cells. RINm5F cells were pre-treated with the indicated concentrations of SPA0355 for 3 h, and interleukin-1β (IL-1β) and interferon-γ (IFN-γ) were added for 30 min. (a) NF-κB-DNA binding was analyzed by electrophoretic mobility shift assay (EMSA). (b) IκB kinase (IKK) phosphorylation and IκBα degradation in the cytosol and nuclear translocation of p65 and p50 were determined by western blotting. β-Actin and proliferating cell nuclear antigen (PCNA) were used as loading controls for cytosolic and nuclear proteins, respectively. The data are representative of three separate experiments.
Effects of SPA0355 on cytokine-induced JAK-STAT activation in RINm5F cells
It has been reported that cytokine-elicited JAK-STAT signaling pathways are involved in cytokine-induced iNOS expression.8 Furthermore, it has also been proposed that STAT activation is specifically involved in IFN-γ-mediated cytotoxicity in β cells.12 We therefore examined the effects of SPA0355 on cytokine-induced STAT activation in RINm5F cells. The results showed that the degree of the phosphorylation of STAT-1 and STAT-3 was increased within 30 min following treatment with cytokines (Figure 3a). Moreover, cytokines also induced nuclear translocation of STAT-1 and STAT-3 proteins (Figure 3b). However, following pre-treatment with SPA0355, cytokine-induced phosphorylation and nuclear translocation of STAT proteins was attenuated.
SPA0355 inhibits cytokine-induced Janus kinase (JAK)-signal transducer and activator of transcription (STAT) activation in RINm5F cells. RINm5F cells were pre-treated with the indicated concentrations of SPA0355 for 3 h, and interleukin-1β (IL-1β) and interferon-γ (IFN-γ) were subsequently added for 30 min. (a) Whole-cell lysates were recovered, and protein levels of suppressor of cytokine signaling (SOCSs), p-STATs and STATs were detected by western blotting. (b) Nuclear fractions were recovered, and translocation of STAT proteins to the nucleus was detected by western blotting. The data are representative of three separate experiments.
Some studies have indicated that the overexpression of SOCS-113 or SOCS-314,15 in pancreatic β cells exerts a protective effect against cytokine toxicity by blocking both the NF-κB and JAK-STAT signaling pathways. We therefore examined whether SOCS-1 and SOCS-3 are involved in our experimental system. We found that both SOCS-1 and SOCS-3 were downregulated in RINm5F cells after a 1-h incubation with cytokines (Figure 3a). However, following pre-treatment with SPA0355, the protein levels of SOCS-1 and SOCS-3 were restored compared with the control. These results indicate that suppression of the JAK-STAT pathway is partially responsible for the SPA0355-induced inhibition of cytokine-induced iNOS expression and subsequent cell death.
Effects of SPA0355 on cytokine-induced damage to islets
We further examined the protective effects of SPA0355 using pancreatic islets isolated from Sprague–Dawley rats. Consistent with the results obtained with RINm5F cells, the degree of cytokine-induced NO production and iNOS expression were reduced to levels similar to those of the control following pre-treatment with SPA0355 (Figure 4a). In addition, following pre-treatment with SPA0355, cytokine-induced NF-κB activation (Figure 4b) and STAT phosphorylation were efficiently blocked (Figure 4c).
SPA0355 inhibits cytokine-induced activation of the nuclear factor κB (NF-κB) and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathways in islets. Rat islets were treated with interleukin-1β (IL-1β) and interferon-γ (IFN-γ) with or without 3-h pre-treatment with 40 μM SPA0355. (a) Nitrite production and inducible nitric oxide synthase (iNOS) mRNA and protein expression levels were determined after 24 h. NF-κB-DNA binding (b) and STAT phosphorylation (c) were determined after 30 min of incubation. The results obtained with triplicate samples are expressed as the mean±s.e.m. (n=9). **P<0.01 vs untreated control; ##P<0.01 vs cytokine treatment.
In the current experimental study, SPA0355 consistently suppressed the cytokine-mediated NF-κB and JAK-STAT pathways, and treatment with SPA0355 resulted in increased islet viability. Upon treatment with cytokines, islets were damaged and weak insulin immunoreactivity was noted (Figure 5A (c and g)). However, following pre-treatment with SPA0355, the effect of cytokines on the islets was blocked. In addition, there were no significant differences with respect to morphological characteristics between control and pre-treated islets based on hematoxylin and eosin staining and immunohistochemistry. The islets had a well-defined margin, a round shape and strong insulin immunoreactivity (Figure 5A (c and h)). These histopathological findings were confirmed using apoptotic staining. Following pre-treatment with SPA0355, the effect of cytokines was blocked, which caused increased resistance to cytokine-induced apoptosis (Figure 5A (l)). To obtain functional data, we examined the efficacy of SPA0355 in preventing cytokine-induced impairment of insulin secretion (Figure 5B). We assayed basal and glucose-stimulated insulin secretion after cytokine exposure. The results showed that the amount of glucose-stimulated insulin secretion was 32.72±0.6 ng mg protein–1 h–1 in control islets and 17.90±0.1 ng mg protein–1 h–1 in cytokine-treated islets. However, following pre-treatment with SPA0355, the degree of cytokine-impaired insulin secretion was restored to a level similar to that of the control. Basal insulin release among the groups was similar.
SPA0355 preserves glucose-stimulated insulin secretion in rat islets. (A) Rat islets were treated with interleukin-1β (IL-1β) and interferon-γ (IFN-γ) with or without a 3-h pre-treatment with 40 μM SPA0355. Following a 48-h incubation, the islets were stained with hematoxylin and eosin (H&E) or labeled using anti-insulin antibodies and subsequently examined by microscopy. Apoptosis was evaluated using the APOPercentage apoptosis assay kit. Apoptotic islets appeared bright pink and are expressed as a percentage of total islets. (B) Glucose-stimulated insulin secretion was quantified by enzyme-linked immunosorbent assay (ELISA) and normalized to total protein content. The results obtained with triplicate samples are expressed as the mean±s.e.m. (n=9). **P<0.01 vs untreated control; ##P<0.01 vs cytokine treatment.
Effects of SPA0355 on diabetes development in NOD mice
Finally, we examined the efficacy of SPA0355 in inhibiting diabetes in NOD mice. To accomplish this, we began injecting NOD mice with SPA0355 before the onset of diabetes (at the age of 8 weeks). In the untreated NOD mice, diabetes occurred beginning at week 13 (Figure 6a). At the age of 33 weeks, 80% of NOD mice became diabetic, and 50% of diabetic mice developed a severe insulitis (Figure 6b). However, in the SPA0355-treated group, 35% of NOD mice developed diabetes and had a slower course of disease progression with less severe insulitis.
SPA0355 attenuates diabetes development in non-obese diabetic (NOD) mice. Female NOD mice were injected intraperitoneally at 2-day intervals with SPA0355 (1 mg kg–1) or corn oil (n=20, each group). Diabetes development was determined by measurement of blood glucose levels (over 300 mg dl–1). (a) The percentage of diabetic mice is shown. (b) Pancreata were isolated from surviving mice at the age of 33 weeks, and pancreas sections were counterstained with hematoxylin and eosin (H&E) or labeled with insulin antibody. Insulitis was scored as described in the Materials and methods section.
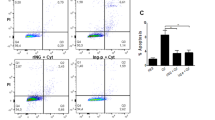
The anti-diabetic effect of SPA0355 in a model of type 1 diabetes was further investigated via adoptive transfer experiments in NOD/SCID mice. Adoptive transfer of splenocytes from diabetic NOD mice induced diabetes in NOD/SCID mice (100%), and SPA0355 treatment reduced the incidence of diabetes to 60% (Figure 7a). To address the mechanism of action of SPA0355 in type 1 diabetes, the activation state of macrophages was determined by CD80 expression and tumor necrosis factor-α (a hallmark cytokine of macrophage activation) production in splenocytes. As shown in Figure 7b, SPA0355 treatment inhibited both macrophage activation and tumor necrosis factor-α production. These results indicate that SPA0355 attenuates spontaneous diabetes mellitus in the NOD mouse model of type 1 diabetes via suppression of macrophage activation.
SPA0355 attenuates diabetes development in non-obese diabetic (NOD)/severe combined immunodeficient (SCID) mice. NOD/SCID mice were intraperitoneally injected with SPA0355 or vehicle at 2-day intervals. After 6 days, splenocytes (1 × 107 cells per mouse) from diabetic NOD mice were adoptively transferred to NOD/SCID mice to induce type 1 diabetes. After adoptive transfer, the treatments were maintained until the end of the experiments. (a) The incidence of diabetes was evaluated (n=10, each group). (b) Splenocytes from vehicle-treated or SPA0355-treated mice (n=3, each group) were isolated and analyzed for macrophage activation. The level of CD80 expression is shown as the mean fluorescence intensity (MFI), and tumor necrosis factor-α (TNF-α) production is shown as the percentage of TNF-α producing cells in splenocytes. The results are expressed as the mean±s.e.m. *P<0.05 vs vehicle group.
Discussion
The current experimental study was conducted to clarify the potential effects of SPA0355, a thiourea derivative, on the development of type 1 diabetes and to determine its molecular mechanism in an experimental model of cytokine- and auto-reactive T-cell-induced damage to pancreatic β cells. Our results show that SPA0355 suppresses cytokine-induced activation of the NF-κB and JAK-STAT signaling pathways and preserves the functional β-cell mass. In addition, SPA0355 attenuates type 1 diabetes development in NOD mice.
Pancreatic β-cell destruction is one of the histopathologic features that are commonly encountered at the onset of type 1 diabetes, and it results from direct contact with islet-infiltrating macrophages and T lymphocytes and/or exposure to their inflammatory products, such as free radicals and cytokines.16 Therefore, any interventions allowing for the preservation of β-cell mass may be novel therapeutic strategies for delaying the progress of type 1 diabetes. Our earlier study indicated that SPA0355 is a small molecule inhibitor of NF-κB that has strong anti-inflammatory effects.10 We therefore selected SPA0355 for this study, and we hypothesized that it may have beneficial effects on the survival and/or function of islets.
Several lines of evidence suggest a central role for NF-κB in the pathogenesis of type 1 diabetes. NF-κB induces multiple pro-inflammatory genes that can contribute to β-cell destruction.6 Several therapeutic attempts at preserving β-cell mass have therefore targeted the NF-κB signaling pathway. Specifically, experimental diabetes models have demonstrated the effectiveness of suppressors of the NF-κB signaling pathway, such as peptide inhibitor of IKK,17 IκBα super-repressor,9 A2018 and flavonoids.19, 20, 21 Our previous study demonstrating the inactivation of NF-κB by SIRT1 also followed this strategy.22 Of the NF-κB signaling molecules, the canonical IKKs are considered the most promising targets in the NF-κB pathway because they are not involved in the activation of any other pathways aside from NF-κB.23 Thus, we examined the efficacy of SPA0355 in triggering IκBα degradation via IKK. Our results showed that SPA0355 decreased the degradation of IκBα in cytokine-treated RINm5F cells, which was evidenced by the levels of phosphorylated IKKα and IKKβ. Of note, there were no changes in the IKKα/β protein levels following treatment with SPA0355. Little is known about the exact mechanisms by which SPA0355 suppresses cytokine-induced IKK phosphorylation. However, our results suggest that inhibition of the IKK complex may be a possible mechanism by which SPA0355 suppresses NF-κB translocation and its action site may be located upstream of IKKα/β.
As NF-κB and STAT are synergistically involved in the regulation of iNOS expression,8, 9 we analyzed the effects of SPA0355 on the JAK-STAT pathway in RINm5F cells and islets. Our results show that SPA0355 suppressed cytokine-induced phosphorylation and nuclear translocation of the STAT-1 protein. By using specific pharmacologic inhibitors, Blanchette et al.24 suggested that JAK2/STAT-1 and extracellular signal-regulated kinase-1/2-dependent pathways were the main factors involved in IFN-γ-inducible iNOS expression. In the JAK-STAT-1 pathway, the expression of IFN-γ regulatory factor-1 is also activated through binding to IFN-γ-stimulated response elements and interaction with NF-κB sites in the iNOS promoter, which would augment IL-1β-induced iNOS expression.25, 26 It has been reported that genetically engineered mice deficient in STAT-1 are completely resistant to IL-1β+IFN-γ-mediated β-cell death in vitro and partially resistant to immune destruction of β cells in vivo.27 Selective inhibition of the JAK-STAT pathway protects β cells from cytokine toxicity11 and prevents the development of insulitis and diabetes in NOD mice.28 Therefore, the suppressive effect of SPA0355 on the activation and nuclear translocation of STAT-1 might contribute to the inhibition of cytokine-induced iNOS expression, β-cell death and islet dysfunction. Of note, SPA0355 also inhibited the activation of STAT-3, another member of the STAT family, which was generally present under baseline conditions to a varying extent.29 The molecular basis of SPA0355-induced STAT-3 inhibition is not clear. However, our results are in agreement with previous reports showing that IFN-γ activates STAT-3 in RINm5F cells.30, 31
STAT proteins are negatively regulated by SOCS proteins. Our results showed that the expression levels of SOCS-1 and SOCS-3 were decreased by cytokine treatment and subsequently restored to levels similar to that of the control following pre-treatment with SPA0355. There was a strong inverse correlation between the degree of STAT expression and that of SOCS proteins. In other words, upon overexpression of SOCS-1, IFN-γ-induced STAT-1 activation and the cytotoxic effects of STAT on β cells are inhibited.13 SOCS-3 can inhibit not only IL-1β- and IFN-γ-induced apoptosis but also NO production through a direct interaction with JAK in INS-1 cells and primary β cells.15, 32 Moreover, SOCS-3 also inhibits IL-1β signaling and gene regulation, at least in part, through inhibition of NF-κB signaling.14 Through self-regulation by the JAK-STAT pathway, SOCS-1 and SOCS-3 define negative feedback loops controlling iNOS expression in response to cytokines.
SPA0355 not only protected RINm5F cells and rat islets against cytokine toxicity but also prevented the occurrence of autoimmune diabetes. SPA0355 protected NOD mice from diabetes development by reducing insulitis. In addition, an adoptive transfer experiment using NOD/SCID mice demonstrated that SPA0355 suppresses macrophage activation in vivo, suggesting an immunomodulatory effect of SPA0355. Previous studies have reported that macrophages have a critical role in type 1 diabetes, and macrophage depletion or inhibition of macrophage activation prevents diabetes development.19, 33, 34 On the basis of our findings in this study, it is possible that SPA0355 inhibits macrophage activation and/or function via manipulation of multiple signaling pathways, including NF-κB and JAK-STAT. As many immune cell types infiltrate the islets of Langerhans and are involved in the destruction of β cells, further studies are needed to examine whether SPA0355 exerts a direct effect on macrophages.
To summarize, we have demonstrated that SPA0355 effectively protects β cells from cytokine-induced injury in vitro, thereby suppressing the occurrence of type 1 diabetes in response to autoimmune attack in vivo. Moreover, we showed a direct correlation between the effectiveness of SPA0355 and the activity of its biochemical target. SPA0355 was well tolerated at the given dose with no evidence of drug toxicity. In addition, our previous in vivo study showed that SPA0355 caused no damage to visceral organs such as the heart, liver and kidney of mice.10 It can therefore be concluded that SPA0355 may be a future therapeutic option that can prevent the destruction of β cells both in the early stages of diabetes onset and after islet transplantation.
References
Gonzalez-Gay MA, Garcia-Unzueta MT, Berja A, Vazquez-Rodriguez TR, Miranda-Filloy JA, Gonzalez-Juanatey C et al. Short-term effect of anti-TNF-α therapy on nitric oxide production in patients with severe rheumatoid arthritis. Clin Exp Rheumatol 2009; 27: 452–458.
Prado CM, Leick-Maldonado EA, Yano L, Leme AS, Capelozzi VL, Martins MA et al. Effects of nitric oxide synthases in chronic allergic airway inflammation and remodeling. Am J Respir Cell Mol Biol 2006; 35: 457–465.
Connelly L, Madhani M, Hobbs AJ . Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: a pro-inflammatory role for eNOS-derived no in vivo. J Biol Chem 2005; 280: 10040–10046.
Beck PL, Li Y, Wong J, Chen CW, Keenan CM, Sharkey KA et al. Inducible nitric oxide synthase from bone marrow-derived cells plays a critical role in regulating colonic inflammation. Gastroenterology 2007; 132: 1778–1790.
Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL . Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005; 54: S97–107.
Eizirik DL, Colli ML, Ortis F . The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Rev Endocrinol 2009; 5: 219–226.
Corbett JA, McDaniel ML . Does nitric oxide mediate autoimmune destruction of β-cells? Possible therapeutic interventions in IDDM. Diabetes 1992; 41: 897–903.
Ganster RW, Taylor BS, Shao L, Geller DA . Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-κB. Proc Natl Acad Sci USA 2001; 98: 8638–8643.
Heimberg H, Heremans Y, Jobin C, Leemans R, Cardozo AK, Darville M et al. Inhibition of cytokine-induced NF-κB activation by adenovirus-mediated expression of a NF-κB super-repressor prevents β-cell apoptosis. Diabetes 2001; 50: 2219–2224.
Lee Y, Hwang J, Lee H, Cheon Y, Ryu J, Lee S et al. SPA0355, a thiourea analogue, inhibits inflammatory responses and joint destruction in fibroblast-like synoviocytes and mice with collagen-induced arthritis. Br J Pharmacol 2011; 164: 794–806.
Lv N, Kim EK, Song MY, Choi HN, Moon WS, Park SJ et al. JANEX-1, a JAK3 inhibitor, protects pancreatic islets from cytokine toxicity through downregulation of NF-κB activation and the JAK/STAT pathway. Exp Cell Res 2009; 315: 2064–2071.
Heitmeier MR, Scarim AL, Corbett JA . Prolonged STAT1 activation is associated with interferon-γ priming for interleukin-1-induced inducible nitric-oxide synthase expression by islets of Langerhans. J Biol Chem 1999; 274: 29266–29273.
Chong MM, Chen Y, Darwiche R, Dudek NL, Irawaty W, Santamaria P et al. Suppressor of cytokine signaling-1 overexpression protects pancreatic β cells from CD8+ T cell-mediated autoimmune destruction. J Immunol 2004; 172: 5714–5721.
Karlsen AE, Heding PE, Frobose H, Ronn SG, Kruhoffer M, Orntoft TF et al. Suppressor of cytokine signalling (SOCS)-3 protects β cells against IL-1β-mediated toxicity through inhibition of multiple nuclear factor-κB-regulated proapoptotic pathways. Diabetologia 2004; 47: 1998–2011.
Karlsen AE, Ronn SG, Lindberg K, Johannesen J, Galsgaard ED, Pociot F et al. Suppressor of cytokine signaling 3 (SOCS-3) protects β-cells against interleukin-1β- and interferon-γ-mediated toxicity. Proc Natl Acad Sci USA 2001; 98: 12191–12196.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012; 35: S64–S71.
Rehman KK, Bertera S, Bottino R, Balamurugan AN, Mai JC, Mi Z et al. Protection of islets by in situ peptide-mediated transduction of the IκB kinase inhibitor Nemo-binding domain peptide. J Biol Chem 2003; 278: 9862–9868.
Grey ST, Arvelo MB, Hasenkamp W, Bach FH, Ferran C . A20 inhibits cytokine-induced apoptosis and nuclear factor κB-dependent gene activation in islets. J Exp Med 1999; 190: 1135–1146.
Lee SM, Yang H, Tartar DM, Gao B, Luo X, Ye SQ et al. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia 2011; 54: 1136–1146.
Song MY, Jeong GS, Kwon KB, Ka SO, Jang HY, Park JW et al. Sulfuretin protects against cytokine-induced β-cell damage and prevents streptozotocin-induced diabetes. Exp Mol Med 2010; 42: 83–88.
Song MY, Kim EK, Moon WS, Park JW, Kim HJ, So HS et al. Sulforaphane protects against cytokine- and streptozotocin-induced β-cell damage by suppressing the NF-κB pathway. Toxicol Appl Pharmacol 2009; 235: 57–67.
Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK et al. Overexpression of SIRT1 protects pancreatic β-cells against cytokine toxicity by suppressing the nuclear factor-κB signaling pathway. Diabetes 2009; 58: 344–351.
Israel A . The IKK complex: an integrator of all signals that activate NF-κB? Trends Cell Biol 2000; 10: 129–133.
Blanchette J, Jaramillo M, Olivier M . Signalling events involved in interferon-γ-inducible macrophage nitric oxide generation. Immunology 2003; 108: 513–522.
Darville MI, Eizirik DL . Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia 1998; 41: 1101–1108.
Paludan SR, Malmgaard L, Ellermann-Eriksen S, Bosca L, Mogensen SC . Interferon (IFN)-γ and Herpes simplex virus/tumor necrosis factor-α synergistically induce nitric oxide synthase 2 in macrophages through cooperative action of nuclear factor-κB and IFN regulatory factor-1. Eur Cytokine Netw 2001; 12: 297–308.
Gysemans CA, Ladriere L, Callewaert H, Rasschaert J, Flamez D, Levy DE et al. Disruption of the γ-interferon signaling pathway at the level of signal transducer and activator of transcription-1 prevents immune destruction of β-cells. Diabetes 2005; 54: 2396–2403.
Cetkovic-Cvrlje M, Dragt AL, Vassilev A, Liu XP, Uckun FM . Targeting JAK3 with JANEX-1 for prevention of autoimmune type 1 diabetes in NOD mice. Clin Immunol 2003; 106: 213–225.
Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004; 53: 2559–2568.
Kim EK, Kwon KB, Song MY, Seo SW, Park SJ, Ka SO et al. Genistein protects pancreatic β cells against cytokine-mediated toxicity. Mol Cell Endocrinol 2007; 278: 18–28.
Matsuda T, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F et al. Silymarin protects pancreatic β-cells against cytokine-mediated toxicity: implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology 2005; 146: 175–185.
Ronn SG, Borjesson A, Bruun C, Heding PE, Frobose H, Mandrup-Poulsen T et al. Suppressor of cytokine signalling-3 expression inhibits cytokine-mediated destruction of primary mouse and rat pancreatic islets and delays allograft rejection. Diabetologia 2008; 51: 1873–1882.
Jun HS, Santamaria P, Lim HW, Zhang ML, Yoon JW . Absolute requirement of macrophages for the development and activation of β-cell cytotoxic CD8+ T-cells in T-cell receptor transgenic NOD mice. Diabetes 1999; 48: 34–42.
Nikolic T, Geutskens SB, van Rooijen N, Drexhage HA, Leenen PJ . Dendritic cells and macrophages are essential for the retention of lymphocytes in (peri)-insulitis of the nonobese diabetic mouse: a phagocyte depletion study. Lab Invest 2005; 85: 487–501.
Acknowledgements
This work was supported by the Bio and Medical Technology Development Program (no. 2012M3A9B2027975), the Basic Science Research Program (no. 2013012280) and by the Medical Research Center Program (no. 2012-0009319 and 2011-0030699) through the National Research Foundation (NRF) funded by the Korean government (MSIP). Additional support was provided by the 2012 Research Fund of Chonbuk National University (to MYS).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Bae, UJ., Song, MY., Jang, HY. et al. The efficacy of SPA0355 in protecting β cells in isolated pancreatic islets and in a murine experimental model of type 1 diabetes. Exp Mol Med 45, e51 (2013). https://doi.org/10.1038/emm.2013.109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2013.109
Keywords
This article is cited by
-
Polyphenols isolated from Broussonetia kazinoki prevent cytokine-induced β-cell damage and the development of type 1 diabetes
Experimental & Molecular Medicine (2015)
-
Transplantation of betacellulin-transduced islets improves glucose intolerance in diabetic mice
Experimental & Molecular Medicine (2014)
-
SPA0355 attenuates ischemia/reperfusion-induced liver injury in mice
Experimental & Molecular Medicine (2014)