Abstract
Growth factor stimulation induces Y783 phosphorylation of phosphoinositide-specific PLC-γ1, and the subsequent activation of this enzyme in a cellular signaling cascade. Previously, we showed that a double point mutation, Y509A/F510A, of PLC-γ1, abolished interactions with translational elongation factor 1-α. Here, we report that the Y509A/F510A mutant PLC-γ1 displayed extremely high levels of Y783 phosphorylation and enhanced catalytic activity, compared to wild-type PLC-γ1, upon treatment of COS7 cells with EGF. In quiescent COS7 cells, the Y509A/F510A mutant PLC-γ1 exhibited a constitutive hydrolytic activity, whereas the wild-type counterpart displayed a basal level of activity. Upon treatment of COS7 cells with EGF, the Y783F mutation in Y509A/F510A PLC-γ1 (Y509A/F510A/Y783F triple mutant) cells also led to an enhanced catalytic activity, whereas Y783F mutation alone displayed a basal level of activity. Our results collectively suggest that the Y509A/F510A mutant is more susceptible to receptor tyrosine kinase-induced Y783 phosphorylation than is wild-type PLC-γ1, but no longer requires Y783 phosphorylation step for the Y509A/F510A mutant PLC-γ1 activation in vivo.
Similar content being viewed by others
Introduction
PLC-γ1 plays a pivotal role in cell growth and differentiation by hydrolyzing phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DG). Overexpression of PLC-γ1 induces malignant transformation in nude mice (Chang et al., 1997) while targeted deletion of PLC-γ1 results in embryonic lethality in mice (Ji et al., 1997). The requirement for protein tyrosine phosphorylation prior to activation of PLC-γ1 is currently widely accepted (Kim et al., 1991; Rhee, 2001; Poulin et al., 2005; Choi et al., 2006). Briefly, agonist stimulation results in activation of receptor tyrosine kinases, such as the EGF receptor and the PDGF receptor, followed by association with PLC-γ1 (Rhee, 2001; Meisenhelder et al., 1989). This interaction allows receptor tyrosine kinases to phosphorylate PLC-γ1 at tyrosine residues, including Y771, Y783, and Y1254 (Kim et al., 1990, 1991; Wahl and Carpenter, 1998; Sekiya et al., 2004; Poulin et al., 2005). Among these residues, Y783 is proposed to be a critical phosphorylation site for enzyme activation, in view of the finding that the Y783F mutant protein is devoid of PIP2 hydrolyzing activity in agonist-treated cells (Kim et al., 1991; Sekiya et al., 2004; Poulin et al., 2005).
PLC-γ1 contains two PH domains for protein-protein and protein-lipid interaction. PH1 is located within the 150 residues closest to the N-terminus, whereas PH2 is located near the center of the molecule; the two domains are separated by the SH2-SH2-SH3 domain (Gibson et al., 1994; Kavran et al., 1998). PH domains bind with high specificity and affinity to PtdInsPs, such as PI(3)P, PI(4)P, PI(5)P, PI(3,4)P2, PI(3,5)P2, PI(4,5)P2, and PI(3,4,5)P3 (Runnels et al., 1996; Wang et al., 1999), and such domains in signaling molecules are often involved in targeted translocation of molecules to cell membranes (Lemmon et al., 1996; Falasca et al., 1998).
A split PH2 domain (nPH2) is located immediately upstream of the N-terminal SH2 of PLC-γ1, and is involved in protein-protein interactions with EF-1α, β-tubulin, and NF-L (Chang et al., 2002, 2005; Kim et al., 2006). In a previous study, we demonstrated, for the first time, that the Y509 and F510 residues within the nPH2 domain were critical for protein-protein interaction, and a double point mutant (Y509A/F510A) completely lost binding affinity with EF-1α (Chang et al., 2002). Based on this finding, DeBell and colleagues (DeBell et al., 2007) further reported that a Y509A/F510A mutant protein modulated cellular calcium signaling and NF-AT activation upon anti-IgM stimulation in a chicken B-cell line, P10-14.
Here, we show that the Y509A/F510A mutation in PLC-γ1 significantly enhances both Y783 phosphorylation and IP3 generation, compared to the activity of the wild-type counterpart, upon EGF stimulation. Interestingly, a different substitution, Y783F, in the Y509A/F510A protein, generates a triple point PLC-γ1 (Y509A/F510A/Y783F) mutant, which is unable to phosphorylate tyrosine at position 783, but retains an increased capacity for inositol phosphate generation to a similar extent as wild-type PLC-γ1 active in EGF-treated COS7 cells. Our results suggest that Y509A/F510A mutant no longer requires Y783 phosphorylation step for enzyme activation in vivo.
Results
EGF promotes Y783 phosphorylation in the Y509A/F510A PLC-γ1 mutant
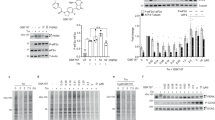
Previous studies by our group focusing on identification of the binding site in the split PH domain of PLC-γ1 revealed that Y509 and F510 were potential regulatory residues of enzyme activity, via mediation of protein-protein association (Chang et al., 2002). Unexpectedly, anti-phosphotyrosine immunoblotting experiments revealed an unusually high level of tyrosine phosphorylation in the Y509A/F510A PLC-γ1 mutant protein (Figure 1A). COS7 cells transfected with FLAG-tagged cDNA encoding either Y509A/F510A mutant or wild-type PLC-γ1 protein were treated with 50 ng/ml EGF for 10 min, or untreated, and cell lysates were subjected to immunoprecipitation using anti-FLAG antibodies. In an anti-phosphotyrosine (monoclonal antibody PY20) immunoblot, the tyrosine phosphorylation level of the mutant was almost five-fold higher than that of wild-type PLC-γ1, as shown by image intensity analysis (Figure 1A).
Tyrosine phosphorylation of the Y509A/F510A mutant PLC-γ1. COS7 cells transfected with wild-type pFLAG-PLC-γ1 (WT) and pFLAG-Y509A/F510A mutant DNA were stimulated with 50 ng/ml EGF for 10 min, or were untreated. Lysates were immunoprecipitated with anti-FLAG M2 antibody. Immunoprecipitated proteins were resolved on 10% (w/v) SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-phosphotyrosine (PY20) (A), anti-pY771 (B), anti-pY783 (C), or anti-pY1254 (D) antibodies, respectively (see above). Each membrane was reprobed with anti-FLAG antibody, to ensure normalization (see below). The diagrams under the immunoblots represent relative image intensities obtained from X-ray film analysis.
Next, we determined the tyrosine residue phosphorylated by the EGF-receptor tyrosine kinase. Resolved proteins, prepared as described above, were immunoblotted using anti-phosphotyrosine-specific antibodies for PLC-γ1. As shown in Figures 1B and 1D, anti-pY771 and anti-pY1254 immunoblots did not reveal increased tyrosine phosphorylation in the Y509A/F510A mutant, whereas Y783 phosphorylation was significantly increased in the double mutant, compared to wild-type PLC-γ1, following EGF treatment (Figure 1C). Our results indicate that the Y509A/F510A mutations strongly promoted Y783 phosphorylation upon EGF stimulation in vivo.
EGF promotes IP3 generation in the Y509A/F510A mutant PLC-γ1
Next, we examined the PIP2-hydrolyzing activity of mutant PLC-γ1 in COS7 cells, in view of the finding that Y783 phosphorylation was a prerequisite for PLC-γ1 activation (Kim et al., 1991; Poulin et al., 2005). For activity analysis, Y509A/F510A mutant and wild-type PLC-γ1 constructs were transfected into COS7 cells, and either treated with EGF for 20 min, or left untreated. As illustrated in Figure 2, the activity of the Y509A/F510A mutant was twice that of wild-type PLC-γ1 in the presence of EGF. In terms of protein expression and IP3 production, activity was increased by half (Figure 2). Interestingly, even in the absence of EGF, Y509A/F510A mutant PLC-γ1 displayed an enhanced IP3 generation capacity, relative to that of EGF-treated wild-type PLC-γ1 (Figure 2). Thus, it appeared that Y509A/F510A mutant PLC-γ1 was constitutively activated in COS7 cells. Our results collectively indicate that the Y509A/F510A mutant has a higher inositol phospholipid-hydrolyzing activity than does wild-type PLC-γ1, in vivo.
Comparison of IP3 production rates in COS7 cells transfected with wild-type and Y509A/F510A mutant PLC-γ1. COS7 cells expressing wild-type or Y509A/F510A mutant PLC-γ1 were labeled with myo-[2-3H] inositol (2 µCi/ml) for 24 h. Labeled cells were pretreated with 20 mM LiCl for 15 min, and further treated with EGF for 20 min, or left untreated. Extracts were loaded onto anion exchange columns. Half of the total inositol phosphate eluted from the column was used for scintillation counting. Western blot images [anti-FLAG immunoblot (above) and anti-β-actin immunoblot (below)] within a box represent the expression levels of mutant proteins, thus including normalization. Data are presented as averages of triplicate determinations (means ± SD), from either of the two experiments, which yielded similar results.
Y509A/Y510A mutant PLC-γ1 does not require Y783 phosphorylation for activation
As the Y509A/F510A mutant displayed a remarkable increase in IP3 generation with respect to its wild-type counterpart, even without EGF stimulation, we addressed the role of Y783 phosphorylation in mutant PLC-γ1 activity. An additional Y783F mutation was introduced into Y509A/F510A PLC-γ1 and the inositol phospholipid-hydrolyzing activity of the triple mutant (Y509A/F510A/Y783F) was examined in vivo. Initially, we compared the phosphorylation status of 783 residue of Y509A/F510A/Y783F with that of the Y509A/F510A mutant by anti-pY783 immunoblotting (Figure 3A). Recombinant constructs were transfected into COS7 cells and immunoprecipitated with an anti-FLAG antibody, followed by immunoblotting with an anti-pY783 antibody. H335Q, a lipase-inactive mutant used as a catalytically inactive control, and wild-type PLC-γ1, displayed enhanced levels of Y783 phosphorylation upon EGF stimulation, whereas no immunoreactive band was observed for the Y509A/F510A/Y783F mutant when anti-pY783 antibody was used as a probe. This suggests that tyrosine phosphorylation at position 783 was completely abolished in the Y509A/F510A/Y783F mutant (Figure 3A). However, as shown above (Figure 1C), Y783 phosphorylation was significantly increased in the Y509A/F510A mutant upon EGF stimulation (Figure 3A). The Y783F PLC-γ1 mutant was not phosphorylated at residue 783, as described elsewhere (data not shown).
Comparison of Y783 phosphorylation and IP3 production of Y509A/F510A and Y509A/F510A/Y783F mutant PLC-γ1 proteins. (A) Wild-type PLC-γ1 and the derivative mutant PLC-γ1, from COS7 cells transfected with pFLAG-PLC-γ1 (WT), -H335Q, -Y509A/F510A, and -Y509A/F510A/Y783F, were immunoprecipitated using anti-FLAG antibody, and resolved proteins were immunoblotted employing an anti-pY783 antibody. (B) COS7 cells expressing the above combinations of DNA were labeled with myo-[2-3H] inositol (2 µCi/ml) for 24 h. Labeled cells were treated with EGF for 20 min, or left untreated. Extracts were applied to an anion exchange column, and half the level of total inositol phosphate eluted from the column used for scintillation counting. Western blot images [anti-FLAG immunoblot (above) and anti-β-actin immunoblot (below)] represent expression levels of mutant proteins, including normalization data. Data are presented as averages of triplicate determinations (means ± SD) from one of two experiments, which yielded similar results.
Subsequently, we compared the IP3-production abilities of the Y509A/F510A and Y509A/F510A/Y783F mutant proteins. The Y509A/F510A/Y783F mutant generated lower levels of total inositol phosphate than did the Y509A/F510A mutant, but the levels were comparable to those created by wild-type PLC-γ1 protein action after EGF stimulation, thus suggesting that Y783 phosphorylation was not critical for enzyme activity in the Y509A/F510A mutant (Figure 3B). Additionally, the Y509A/F510A mutant protein produced increased amounts of IP3 (approximately two-fold higher than that after action of wild-type PLC-γ1) in quiescent COS7 cells, whereas vector-transfected control and wild-type PLC-γ1-transfected cells produced basal levels of IP3 in the absence of EGF stimulation (Figure 3B). Based on these results, we would propose that Y783 phosphorylation is important for wild-type PLC-γ1 activation, but is not a prerequisite for activation of the Y509A/F510A mutant protein. Moreover, Y509A/F510A PLC-γ1 was constitutively active in vivo. Thus, the split half of the PH domain, located immediately upstream of the N-terminal SH2 of PLC-γ1, which contains Y509 and F510, may be key in elucidation of the PLC-γ1 activation mechanism.
Discussion
Activated PLC-γ1 produces second messengers, including diacylglycerol and IP3, and is thus a key enzyme in cell signaling. PLC-γ1, a relatively large molecule consisting of 1,290 amino acids, is structurally arranged into two PH domains, two SH2 domains, one SH3 domain, and catalytic X and Y domains. Details of the mechanism underlying PLC-γ1 activation remain to be established.
To date, the preferred model suggested for the activation mechanism is Y783 phosphorylation that may result in conformational changes within the molecule (Poulin et al., 2005). Phosphorylated PLC-γ1 intramolecularly binds the C-terminal SH2 domain, promoting higher accessibility to the substrate, PIP2 (DeBell et al., 1999, 2007; Poulin et al., 2005). Data of our present study show that the Y509A/F510A mutant protein exhibits a remarkable increase in PIP2-hydrolyzing activity, without a need for Y783 phosphorylation. H335Q PLC-γ1 was previously identified as a lipase-inactive mutant (Smith et al., 1994; Huang et al., 1995). However, no in vivo lipase-active mutants have been reported to date. Thus, we generated a constitutively active mutant, PLC-γ1, which may be useful for clarification of the PLC-γ1 activation mechanism.
The Y509A/F510A mutant displayed a robust increase in Y783 phosphorylation, compared to wild-type PLC-γ1, following EGF treatment (Figure 1C). The mutant protein additionally exhibited a higher IP3 production rate compared to that achieved by the wild-type counterpart (Figure 2). The Y783 phosphorylation level was proportional to IP3 production rate, consistent with what was noted with wild-type PLC-γ1, as reported previously (Meisenhelder et al., 1989; Kim et al., 1990, 1991; Wahl and Carpenter, 1998; Rhee, 2001; Sekiya et al., 2004; Poulin et al., 2005; Choi et al., 2006). However, the most interesting finding with respect to the enzyme activation mechanism is that the Y509A/F510 mutant produced higher levels of IP3 than did wild-type protein, even in the absence of EGF stimulation (Figures 2 and 3B). Y783 phosphorylation is generally considered to be necessary for PLC-γ1 activation. Our results show that the Y509A/F510A/Y783F mutant retained PIP2-hydrolyzing activity, to a similar extent as shown by wild-type protein, conclusively indicating that Y783 phosphorylation is unnecessary for Y509A/F510A PLC-γ1 activation, and supporting constitutive activation of the mutant protein in vivo (Figure 3B).
In terms of the PLC-γ1 activation mechanism, we propose that conformational changes occur in the split PH domain of the Y509A/F510A mutant. Both tyrosine and phenylalanine within the split PH domain are aromatic residues responsible for hydrophobic interactions. Thus, the overall structure in the region of the split PH domain may be disrupted by substitution of both amino acids with neutral residues, such as alanine. So the Y509A/F510A mutation of the split PH domain may result in increased substrate accessibility. In a previous study by our group, the Y509A/F510A mutant was shown to lose binding affinity for EF-1α, but retained an affinity for inositol phospholipid (Chang et al., 2002; Kim et al., 2004). Moreover, assay of the enzyme activity of the purified Y509A/F510A mutant protein, in sonicated micelles in vitro, revealed no change in PIP2 hydrolysis activity (Chang et al., 2002). In contrast, our current results show that the Y509A/F510A mutant protein produced elevated levels of IP3, both in the presence and absence of agonist in vivo. When it is considered that PIP2 hydrolysis by inactive PLC-γ1 is inhibited in quiescent cells by effective removal of substrate via intramolecular interaction (Poulin et al., 2005; DeBell et al., 2007), a possible explanation for the current finding is that the Y509A/F510A mutation results in an open-state conformation that retains activity with respect to substrate hydrolysis. This theory is supported by the existence of a PLC catalytic-inhibitory peptide (PCI peptide) encoded immediately downstream of the C-terminal SH2 domain of PLC-γ1 (Homma and Takenawa, 1992; Homma et al., 1997). For example, the Y509A/F510A mutation may induce a conformational change, leading to concealment of the PCI peptide within the molecule.
Following growth factor stimulation, inactive PLC-γ1 translocates from the cytosol to the membrane where PLC-γ1 is activated to hydrolyze membrane-anchored inositol phospholipid (Falasca et al., 1998; Rhee, 2001). So it is acceptable that the Y509A/F510A/Y783F mutant PLC-γ1 shows an increased PIP2-hydrolyzing activity upon EGF treatment. Following EGF stimulation, active form of the Y509A/F510A/Y783F mutant protein (already activated by mutations of both Y509 and F510 residues) just translocates from the cytosol to the membrane to access PIP2. Whereas no direct evidence for structural delineation of Y509A/F510A activation is yet available, further experiments with our mutant protein may facilitate an understanding of the PLC-γ1 activation mechanism.
Methods
Antibodies and cell culture
Monoclonal and polyclonal anti-PLC-γ1 antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Polyclonal anti-phospho Tyr-771, Tyr-783 and Tyr-1254 antibodies have been described previously (Bae et al., 2002). The monoclonal anti-FLAG antibody and EGF were purchased from Sigma-Aldrich (St. Louis, MO). HRP-conjugated goat anti-mouse and goat anti-rabbit antibodies were acquired from Upstate Inc. (Lake Placid, NY). COS7 cells were cultured in DMEM containing 10% FBS and 100 U penicillin-streptomycin at 37℃.
Vector constructs
Rat cDNAs encoding wild-type PLC-γ1 (Suh et al., 1988), Y509A/F510A (Chang et al., 2002), and Y509A/F510A/Y783F mutant proteins were inserted into the Xba I site of the pFLAG-CMV-2 vector (Sigma-Aldrich, St. Louis, MO) for expression in COS7 cells. All constructs were prepared using the Qiagen Plasmid Maxi Kit (Qiagen Inc., Santa Clarita, CA), and sequences were confirmed by DNA sequencing of the ligation and mutation sites. COS7 cells were transfected with Lipofectamine reagent (Gibco BRL, Gaithersburg, MD). At 48 h after transfection, cells were treated with 50 ng/ml EGF for 20 min or left untreated, and harvested for immunoprecipitation.
Immunoblotting and immunoprecipitation
COS7 cells transfected with plasmids encoding FLAG-tagged PLC-γ1 constructs were washed twice with PBS and lysed with radio immunoprecipitation assay (RIPA) buffer (20 mM HEPES, pH 7.2, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 1 mM sodium orthovanadate, 10 µg/ml leupeptin, 10 µg/ml aprotinin and 1 mM PMSF). For agonist stimulation, cells were serum-starved for 30 h, followed by treatment with 50 ng/ml EGF for 10-20 min. Lysate supernatants were precleared by incubation with anti-FLAG M2 affinity gel (Sigma-Aldrich) for 30 min. Precleared cell lysates were incubated for 2 h with anti-FLAG antibodies conjugated with 50 µl of a 50% slurry of anti-FLAG M2 affinity gel. Immune complexes were collected by centrifugation, washed three times with ice-cold RIPA buffer, and resolved with 10% SDS-PAGE, followed by blotting on to a PVDF membrane. The blot was probed with anti-FLAG or anti-phospho-specific antibodies, and immunoreactive bands visualized by ECL detection using HRP-conjugated goat anti-mouse IgG.
Measurement of inositol phosphate generation in COS7 cells
COS7 cells expressing Y509A/F10A mutant or wild-type PLC-γ1 were seeded at a density of 5 × 105 cells into 60 mm dishes, and cultured for 2 days. Cells were labeled with myo-[2-3H] inositol (2 µCi/ml) in inositol-free DMEM for 24 h. Subsequently, labeled cells were washed with PBS, and pretreated with 20 mM LiCl for 15 min in bicarbonate-free DMEM containing 20 mM HEPES (pH 7.2) and 1 mg/ml of bovine serum albumin. Stimulation was initiated by addition of the indicated concentrations of EGF for 20 min, and terminated with 500 µl of ice-cold 5% HClO4. After 30 min in an ice bath, extracts were centrifuged, diluted (1:5) with distilled water, and applied to a Bio-Rad Dowex AG1-X8 anion exchange column. The column was washed with 10 ml of distilled water, followed by 10 ml of 0.06 M sodium tetraborate. Total inositol phosphates were eluted with a solution containing 1 M ammonium formate and 0.1% formic acid. Half of the eluted volume was subjected to scintillation counting for estimating the quantity of inositol phosphate.
Abbreviations
- IP3:
-
inositol 1,4,5-trisphosphate
- PIP2:
-
phosphatidylinositol 4,5-bisphosphate
- RIPA:
-
radio immunoprecipitation assay
References
Bae SS, Choi JH, Oh YS, Yun SU, Ryu SH, Suh P-G . Regulation of phospholipase C-gamma1 by protein kinase A-dependent phosphorylation . Adv Enzyme Regul 2002 ; 42 : 195 - 211
Chang J-S, Noh DY, Park IA, Kim MJ, Song H, Ryu SH, Suh P-G . Overexpression of phospholipase C-gamma1 in rat 3Y1 fibroblast cells leads to malignant transformation . Cancer Res 1997 ; 57 : 5465 - 5468
Chang J-S, Seok H, Kwon TK, Min DS, Ahn BH, Lee YH, Seo JW, Kim JW, Iwashita S, Omori A, Ichinose S, Numata O, Seo JK, Oh YS, Suh P-G . Interaction of elongation factor-1alpha and pleckstrin homology domain of phospholipase C-gamma 1 with activating its activity . J Biol Chem 2002 ; 277 : 19697 - 19702
Chang J-S, Kim S-K, Kwon TK, Bae SS, Min DS, Lee YH, Kim SO, Seo JK, Choi JH, Suh P-G . Pleckstrin homology domains of phospholipase C-gamma1 directly interact with beta-tubulin for activation of phospholipase C-gamma1 and reciprocal modulation of beta-tubulin function in microtubule assembly . J Biol Chem 2005 ; 280 : 6897 - 6905
Choi JH, Kim HS, Kim SH, Yang YR, Bae YS, Chang J-S, Kwon HM, Ryu SH, Suh P-G . Phospholipase Cgamma1 negatively regulates growth hormone signalling by forming a ternary complex with Jak2 and protein tyrosine phosphatase-1B . Nat Cell Biol 2006 ; 8 : 1389 - 1397
DeBell KE, Stoica BA, Veri MC, Di Baldassarre A, Miscia S, Graham LJ, Rellahan BL, Ishiai M, Kurosaki T, Bonvini E . Functional independence and interdependence of the Src homology domains of phospholipase C-gamma1 in B-cell receptor signal transduction . Mol Cell Biol 1999 ; 19 : 7388 - 7398
DeBell K, Graham L, Reischl I, Serrano C, Bonvini E, Rellahan B . Intramolecular regulation of phospholipase C-gamma1 by its C-terminal Src homology 2 domain . Mol Cell Biol 2007 ; 27 : 854 - 863
Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J . Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting . EMBO J 1998 ; 17 : 414 - 422
Gibson TJ, Hyvönen M, Musacchio A, Saraste M, Birney E . PH domain: the first anniversary . Trends Biochem Sci 1994 ; 19 : 349 - 353
Homma MK, Yamasaki M, Ohmi S, Homma Y . Inhibition of phosphoinositide hydrolysis and cell growth of Swiss 3T3 cells by myristoylated phospholipase C inhibitor peptides . J Biochem 1997 ; 122 : 738 - 742
Homma Y, Takenawa T . Inhibitory effect of src homology (SH) 2/SH3 fragments of phospholipase C-gamma on the catalytic activity of phospholipase C isoforms. Identification of a novel phospholipase C inhibitor region . J Biol Chem 1992 ; 267 : 21844 - 21849
Huang PS, Davis L, Huber H, Goodhart PJ, Wegrzyn RE, Oliff A, Heimbrook DC . An SH3 domain is required for the mitogenic activity of microinjected phospholipase C-gamma 1 . FEBS Lett 1995 ; 358 : 287 - 292
Ji QS, Winnier GE, Niswender KD, Horstman D, Wisdom R, Magnuson MA, Carpenter G . Essential role of the tyrosine kinase substrate phospholipase C-gamma1 in mammalian growth and development . Proc Natl Acad Sci USA 1997 ; 94 : 2999 - 3003
Kavran JM, Klein DE, Lee A, Falasca M, sakoff SJ, Skolnik EY, Lemmon MA . Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains . J Biol Chem 1998 ; 273 : 30497 - 30508
Kim HK, Kim JW, Zilberstein A, Margolis B, Kim JG, Schlessinger J, Rhee SG . PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma1 phosphorylation on tyrosine residues 783 and 1254 . Cell 1991 ; 65 : 435 - 441
Kim JW, Sim SS, Kim UH, Nishibe S, Wahl MI, Carpenter G, Rhee SG . Tyrosine residues in bovine phospholipase C-gamma phosphorylated by the epidermal growth factor receptor in vitro . J Biol Chem 1990 ; 265 : 3940 - 3943
Kim S-K, Wee SM, Chang J-S, Kwon TK, Min DS, Lee YH, Suh P-G . Point mutations in the split PLC-gamma1 PH domain modulate phosphoinositide binding . J Biochem Mol Biol 2004 ; 37 : 720 - 725
Kim S-K, Choi JH, Suh PG, Chang J-S . Pleckstrin homology domain of phospholipase C-gamma1 directly binds to 68-kDa neurofilament light chain . Exp Mol Med 2006 ; 38 : 265 - 272
Lemmon MA, Ferguson KM, Schlessinger J . PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface . Cell 1996 ; 85 : 621 - 624
Meisenhelder J, Suh PG, Rhee SG, Hunter T . Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro . Cell 1989 ; 57 : 1109 - 1122
Poulin B, Sekiya F, Rhee SG . Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-gamma1 . Proc Natl Acad Sci USA 2005 ; 102 : 4276 - 4281
Rhee SG . Regulation of phosphoinositide-specific phospholipase C . Annu Rev Biochem 2001 ; 70 : 281 - 312
Runnels LW, Jenco J, Morris A, Scarlata S . Membrane binding of phospholipases C-beta 1 and C-beta 2 is independent of phosphatidylinositol 4,5-bisphosphate and the alpha and beta gamma subunits of G proteins . Biochemistry 1996 ; 35 : 16824 - 16832
Sekiya F, Poulin B, Kim YJ, Rhee SG . Mechanism of tyrosine phosphorylation and activation of phospholipase C-gamma 1. Tyrosine 783 phosphorylation is not sufficient for lipase activation . J Biol Chem 2004 ; 279 : 32181 - 32190
Smith MR, Liu YL, Matthews NT, Rhee SG, Sung WK, Kung HF . Phospholipase C-gamma 1 can induce DNA synthesis by a mechanism independent of its lipase activity . Proc Natl Acad Sci USA 1994 ; 91 : 6554 - 6558
Suh P-G, Ryu SH, Moon KH, Suh HW, Rhee SG . Cloning and sequence of multiple forms of phospholipase C . Cell 1988 ; 54 : 161 - 169
Wahl M, Carpenter G . Regulation of epidermal growth factor-stimulated formation of inositol phosphates in A-431 cells by calcium and protein kinase C . J Biol Chem 1998 ; 263 : 7581 - 7590
Wang T, Pentyala S, Rebecchi MJ, Scarlata S . Differential association of the pleckstrin homology domains of phospholipases C-beta 1, C-beta 2, and C-delta 1 with lipid bilayers and the beta gamma subunits of heterotrimeric G proteins . Biochemistry 1999 ; 38 : 1517 - 1524
Acknowledgements
This work was supported by a Grant from the Daejin University (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chung, SH., Kim, SK., Kim, J. et al. A double point mutation in PCL-γ1 (Y509A/F510A) enhances Y783 phosphorylation and inositol phospholipid-hydrolyzing activity upon EGF stimulation. Exp Mol Med 42, 216–222 (2010). https://doi.org/10.3858/emm.2010.42.3.023
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2010.42.3.023
Keywords
This article is cited by
-
Na+,K+-ATPase as a docking station: protein–protein complexes of the Na+,K+-ATPase
Cellular and Molecular Life Sciences (2013)