Abstract
4-1BB, a member of the tumor necrosis factor receptor (TNFR) superfamily, is a major costimulatory receptor that is rapidly expressed on the surface of CD4+ and CD8+ T cells after antigen- or mitogen-induced activation. The interaction of 4-1BB with 4-1BBL regulates immunity and promotes the survival and expansion of activated T cells. In this study, the expression of 4-1BB and 4-1BBL was examined during regeneration of the murine thymus following acute cyclophosphamide-induced involution. Four-color flow cytometry showed that 4-1BB and 4-1BBL were present in the normal thymus and were preferentially expressed in the regenerating thymus, mainly in CD4+CD8+ double-positive (DP) thymocytes. Furthermore, the CD4loCD8lo, CD4+CD8lo and CD4loCD8+ thymocyte subsets, representing stages of thymocyte differentiation intermediate between DP and single-positive (SP) thymocytes, also expressed 4-1BB and 4-1BBL during thymus regeneration but to a lesser degree. Interestingly, the 4-1BB and 4-1BBL positive cells among the CD4+CD8+ DP thymocytes present during thymus regeneration were TCRhi and CD69+ unlike the corresponding controls. Moreover, the 4-1BB and 4-1BBL positive cells among the intermediate subsets present during thymus regeneration also exhibited TCRhi/int and CD69+/int phenotypes, indicating that 4-1BB and 4-1BBL are predominantly expressed by the positively selected population of the CD4+CD8+ DP and the intermediate thymocytes during thymus regeneration. RT-PCR and Western blot analyses confirmed the presence and elevated levels of 4-1BB and 4-1BBL mRNA and protein in thymocytes during thymus regeneration. We also found that the interaction of 4-1BB with 4-1BBL promoted thymocyte adhesion to thymic epithelial cells. Our results suggest that 4-1BB and 4-1BBL participate in T lymphopoiesis associated with positive selection during recovery from acute thymic involution.
Similar content being viewed by others
Introduction
The 4-1BB receptor (also referred to as CD137, ILA and TNFRSF9), is a member of the low affinity nerve growth factor receptor/tumor necrosis factor receptor (NGFR/TNFR) family of integral type I membrane protein. It is a potent T cell costimulatory receptor induced primarily by activated T cells (Kwon and Weissman, 1989; Pollok et al., 1993). A high affinity ligand for 4-1BB (4-1BBL; also called CD137L and TNFSF9), a type II membrane protein of the TNF superfamily, has been found to be mainly expressed on activated antigen-presenting cells such as dendritic cells (DCs), B cells, and macrophages (Goodwin et al., 1993; Laderach et al., 2003; Schwarz, 2005).
The costimulatory ability of 4-1BB is of importance in that 4-1BB on its own can provide a costimulatory signal that activates resting T cells independently of other costimulatory molecules such as the CD28, even though signaling through CD28 has been shown to be critical for activation of T cells, and CD28 is widely considered the primary T cell receptor delivering costimulatory signals to resting T cells (Lenschow et al., 1996; Saoulli et al., 1998; Sica and Chen, 2000; Croft, 2003; Watts, 2005). Costimulation through 4-1BB by either 4-1BBL or agonistic monoclonal antibodies (mAbs) enhances T cell activity (Pollok et al., 1993; Hurtado et al., 1997), promotes CD8+ T cell survival (Takahashi et al., 1999), eradicates established tumors (Melero et al., 1997; Ye et al., 2002), promotes rejection of cardiac and skin allografts (Cho et al., 2004), broadens primary antiviral CD8+ T cell responses (Halstead et al., 2002), enhances the memory pool of antigen-specific CD8+ T cells (Bertram et al., 2002), and increases T cell cytolytic potential (Shuford et al., 1997). In addition, 4-1BB-mediated signals suppress CD4+ T cell responses (Mittler et al., 1999) and ameliorate both antigen-induced organ-specific autoimmune diseases such as experimental autoimmune encephalomyelitis (Sun et al., 2002b) and experimental autoimmune rheumatoid arthritis (Seo et al., 2004), and spontaneous systemic autoimmune diseases such as systemic lupus eythematosus (Sun et al., 2002a; Foell et al., 2003). The mechanisms underlying these immunosuppressive effects are not yet fully understood, but it has been shown that 4-1BB-mediated suppression of rheumatoid arthritis is caused by antigen-dependent induction of CD11c+CD8+ T cells that produce IFN-γ, which suppresses antigen-specific CD4+ T cells by an indoleamine 2,3-dioxygenase-dependent mechanism (Seo et al., 2004). Importantly, the 4-1BB receptor/ligand system, like other members of the TNF-α systems, can signal in a bidirectional manner. Therefore, activation signals may be initiated not only in the cells that express the receptor but also in the cells bearing the 4-1BBL, e.g., the DCs (Tan et al., 1999; May et al., 2002; Lippert et al., 2008). These findings establish an important role for 4-1BB/4-1BBL in the immune regulation.
Despite these facts that 4-1BB and 4-1BBL play critical roles in T cell function, little is known about the characteristics and function of 4-1BB and 4-1BBL in the thymus, the central lymphoid organ for the development of bone marrow-derived precursor cells into mature T cells. Thus, the aim of the present study was to investigate the expression of 4-1BB and 4-1BBL using a mouse thymus regeneration model in which mature T cells are actively produced from bone marrow-derived precursor cells through the orchestrated processes of T cell development, and to shed light on the role of 4-1BB and 4-1BBL in the thymus.
Results
Presence of 4-1BB and 4-1BBL in the thymocytes and their up-regulation during thymus regeneration
We first tested for expression of 4-1BB and 4-1BBL in control mouse thymocytes by RT-PCR and Western blotting, and detected a low level of expression of both components. Interestingly, their expression was strongly up-regulated during thymus regeneration after cyclophosphamide-induced acute thymic involution (Figures 1A and 1B). In addition, flow cytometric analysis of 4-1BB and 4-1BBL expression demonstrated that their expression during regeneration was regulated in a time-dependent manner (Figures 2A and 2B). The proportion of 4-1BB-expressing thymocytes peaked on day 5 (Figure 2A), and similar kinetics were observed for 4-1BBL-expression (Figure 2B). A significant decrease in the total number of thymocytes was also observed in cyclophosphamide-treated mice during thymus regeneration (Table 1).
Expression of 4-1BB and 4-1BBL mRNA and protein in mouse thymocytes during thymus regeneration. B6 mice (8-10-week-old) were given a single intraperitoneal dose of cyclophosphamide (400 mg/kg body weight), and were killed in groups of four after 2 or 4 days. (A) RT-PCR analysis of 4-1BB, 4-1BBL and GAPDH in thymocytes from control mice (Cont) and 2 days after cyclophosphamide treatment (2d). Gene expression levels were determined after normalization to GAPDH. (B) Western blot analysis of 4-1BB, 4-1BBL and α-tubulin in thymocytes from control (Cont) and 4 days after cyclophosphamide treatment (4d). Data are expressed as ratios of 4-1BB and 4-1BBL protein normalized to α-tubulin protein. Band densities were measured by scanning densitometry and expressed as means + SD. Data are representative of three independent experiments with four or more animals in each group.
Flow cytometric analysis of the expression of 4-1BB and 4-1BBL in total thymocytes during thymus regeneration. B6 mice were killed in groups of four at 1, 2, 3, 4, 5, 6, 7 and 10 days after injection of cyclophosphamide, and thymocytes were stained with biotinylated anti-4-1BB and biotinylated anti-4-1BBL, followed by PE-streptavidin. Percentages of 4-1BB (A) and 4-1BBL (B) positive cells among total thymocytes were calculated. *P < 0.05, by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. Data are means + SD of more than three independent experiments with four or more animals in each group.
Expression of 4-1BB and 4-1BBL is strongly up-regulated in CD4+CD8+ DP thymocytes during thymus regeneration
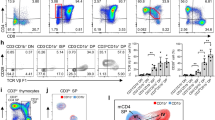
To identify the cell types which express 4-1BB and 4-1BBL, we explored the expression of 4-1BB and 4-1BBL in the thymocyte subsets from control and regenerating thymus by flow cytometry. We first investigated the conventional four thymocyte subsets. In the normal mouse thymus, CD4-CD8- double-negative (DN) and CD4+CD8+ double-positive (DP) thymocytes barely expressed 4-1BB, whereas CD4+ single-positive (SP) and CD8+ SP thymocytes expressed 4-1BB weakly (Figure 3A). Similarly, in the normal mouse thymus, CD4-CD8-DN, CD4+CD8+ DP and CD4+ SP thymocytes barely expressed 4-1BBL, whereas and CD8+ SP thymocytes expressed 4-1BBL weakly (Figure 3B). Interestingly, we observed marked up-regulation of 4-1BB and 4-1BBL expression in the DP thymocytes during thymus regeneration, peaking around day 5 (Figures 3A and 3B). CD8+ SP thymocytes also showed significantly enhanced expression of 4-1BBL during thymus regeneration, but to a much lesser degree (Figure 3B). The CD4+ SP thymocytes expressed a slightly higher level of 4-1BB than of 4-1BBL throughout the entire period of thymus regeneration but their expression did not change during regeneration (Figures 3A and 3B). The CD4-CD8- DN thymocytes barely expressed 4-1BB or 4-1BBL during regeneration (Figures 3A and 3B). In addition, we noted that cyclophosphamide-treated mice during thymus regeneration exhibited a significant decrease in the number of DP thymocytes but showed a significant increase in the number of DN, CD4+ SP and CD8+ SP thymocytes relative to control mice (Figure 3C).
Expression of 4-1BB (A) and 4-1BBL (B) in the four major thymocyte subsets during thymus regeneration. (C) The phenotypic distribution of the four thymocyte subsets during thymus regeneration. Groups of four B6 mice were killed 1, 2, 3, 4, 5, 6, 7 and 10 days after injection of cyclophosphamide. Thymocytes were stained with Pacific Blue-anti-CD4, APC-Cy7-anti-CD8, biotin-anti-4-1BB, biotin-anti-4-1BBL and PE-streptavidin. Data are means + SD of at least three independent experiments with four or more animals per group. *P <0.05 by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test.
4-1BB and 4-1BBL are expressed predominantly in CD4+CD8+TCRhi, CD4loCD8loTCRint, CD4+CD8loTCRhi/int and CD4loCD8+TCRhi/int thymocyte subsets during thymus regeneration
To further characterize these cell populations which express 4-1BB and 4-1BBL, the thymocytes were divided into nine subpopulations on the basis of CD4 and CD8 fluorescence intensities, classified as -, lo and + (Figure 4). There were thus nine categories: CD4-CD8-, CD4loCD8-, CD4-CD8lo, CD4+CD8+, CD4loCD8lo, CD4+CD8lo, CD4loCD8+, CD4+CD8- and CD4-CD8+ thymocytes (Figure 4A). In addition, to define their maturational status, we assessed TCR expression in these subsets. In the normal thymus, this classification yielded the following subsets: CD4-CD8-TCR-, CD4loCD8-TCR-, CD4-CD8loTCR-/int, CD4+CD8+TCR-/int, CD4loCD8loTCR-/int, CD4+CD8loTCR-/int/hi, CD4loCD8+TCR-/int/hi, CD4+CD8-TCRhi and CD4-CD8+TCRhi (Figure 4B). Of interest, TCR expression increased significantly in some of these subpopulations during thymus regeneration, peaking around day 5 after cyclophosphamide treatment. On day 5, these nine subsets exhibited the following phenotypes: CD4-CD8-TCR-, CD4loCD8-TCR-/int, CD4-CD8loTCR-/int, CD4+CD8+TCRhi, CD4loCD8loTCRint, CD4+CD8loTCRint/hi, CD4loCD8+TCRint/hi, CD4+CD8-TCR-/int/hi and CD4-CD8+TCR-/int/hi (Figure 4B). The level of TCR expression in the DP thymocyte subpopulation, in particular, increased markedly (Figure 4B). The CD4loCD8lo, CD4+CD8lo and CD4loCD8+ thymocyte subsets, belonging to the intermediate stage of thymocyte differentiation from DP to SP thymocytes, also displayed intermediate to high levels of TCR expression but to a lesser degree than the CD4+CD8+ DP population (Figure 4B).
Upregulation of TCR expression in the CD4+CD8+ DP and intermediate thymocyte subsets during thymus regeneration. Thymocytes were stained with Pacific Blue-anti-CD4, APC-Cy7-anti-CD8, and FITC-anti-TCRβ. (A) Nine thymocyte subsets were defined on the basis of CD4 and CD8 fluorescence intensities: CD4-CD8-, CD4loCD8-, CD4-CD8lo, CD4+CD8+, CD4loCD8lo, CD4+CD8lo, CD4loCD8+, CD4+CD8-, and CD4-CD8+ thymocytes. (B) Expression of TCRβ was analyzed by flow cytometry in the nine thymocyte subsets during thymus regeneration. Levels of TCRβ expression in thymocytes of control B6 mice (Cont) and of mice 5 days after cyclophosphamide treatment (5d) are shown. Data are representative of more than three independent experiments with four or more animals in each group.
We also assessed TCR expression in gated 4-1BB and 4-1BBL positive DP, CD4loCD8lo, CD4+CD8lo and CD4loCD8+ thymocytes during thymus regeneration. The level of TCR expression was high in the DP thymocytes, intermediate to high in CD4+CD8lo and CD4loCD8+ thymocytes, and intermediate in CD4loCD8lo thymocytes (Figure 5A). A similar pattern was seen for 4-1BBL (Figure 5B).
TCR expression in 4-1BB and 4-1BBL positive CD4+CD8+ DP and intermediate thymocytes during thymus regeneration. Thymocytes were stained with Pacific Blue-anti-CD4, APC-Cy7-anti-CD8, and FITC-anti-TCRβ. The expression of TCRβ was analyzed by flow cytometry in the CD4+CD8+ thymocytes (Cont CD4+CD8+) from normal B6 mice, and in gated 4-1BB (A) and 4-1BBL (B) positive cells in the total (5d total), CD4+CD8+ (5d CD4+CD8+), CD4loCD8lo (5d CD4loCD8lo), CD4+CD8lo (5d CD4+CD8lo), and CD4loCD8+ (5d CD4loCD8+) thymocytes from mice 5 days after cyclophosphamide treatment. Data are representative of at least three independent experiments with four or more animals per group.
Next, we examined the expression of 4-1BB and 4-1BBL in the nine subpopulations by flow cytometry. The majority of thymocytes in the normal mouse thymus barely expressed 4-1BB and 4-1BBL (Figures 6A and 6B). The proportion of 4-1BB positive cells in CD4+CD8+ DP thymocytes increased markedly during thymus regeneration and there were also smaller increase in the intermediate thymocyte subsets (Figure 6A). The expression of 4-1BBL in these nine thymocyte subpopulations exhibited essentially the same pattern (Figure 6B). However, there was no significant change in the number of CD8+ SP thymocytes that expressed 4-1BBL. This contrasted with the situation in the CD8+ SP thymocytes in the original thymocyte subsets, and thus indicated that there was no real increase in 4-1BBL expression in CD8+ SP thymocytes during thymus regeneration (Figures 3B and 6B).
Upregulated expression of 4-1BB and 4-1BBL in the CD4+CD8+ DP and intermediate thymocytes during thymus regeneration. B6 mice were killed in groups of four at 1, 3, 5, 7 and 10 days after injection of cyclophosphamide. They were stained with Pacific Blue-anti-CD4, APC-Cy7-anti-CD8, biotin- anti-4-1BB, biotin-anti-4-1BBL, and PE-streptavidin, and expression of 4-1BB (A) and 4-1BBL (B) was analyzed by flow cytometry in the nine thymocyte subsets. Data are the means + SD of at least three independent experiments with four or more animals in each group. *P < 0.05, **P < 0.01 and ***P < 0.001, by one-way analysis of variance (ANOVA) followed by Tukey's post hoc test.
4-1BB and 4-1BBL are preferentially expressed by positively selected thymocytes in the CD4+CD8+, CD4loCD8+, CD4+CD8lo and CD4loCD8lo thymocyte subsets during thymus regeneration
The above findings indicate that 4-1BB and 4-1BBL are preferentially expressed in CD4+CD8+ DP and the intermediate thymocytes during thymus regeneration, and that these thymocytes show a high level of TCR expression during thymus regeneration. This raised the question as to whether 4-1BB and 4-1BBL expression in these thymocyte populations is related to positive selection. To address this issue, we observed CD69 expression on these thymocytes during thymus regeneration. Only a small percentage of DP thymocytes expressed CD69 in the normal thymus but this number increased dramatically during thymus regeneration (Figure 7A). Next, in order to see whether pre- and post-selected thymocytes differ in the expression of 4-1BB and 4-1BBL, we compared CD69 expression in the 4-1BB and 4-1BBL positive and negative thymocytes during thymus regeneration (Figures 7B and 7C). We indeed found a strong correlation between 4-1BB and 4-1BBL expression and CD69 expression (Figures 7B and 7C). There was also a good correlation between 4-1BB and 4-1BBL expression and CD69 expression in the intermediate thymocytes (Figures 7B and 7C).
CD69 expression in the 4-1BB and 4-1BBL positive CD4+CD8+ DP and intermediate thymocytes during thymus regeneration. Thymocytes were stained with Pacific Blue-anti-CD4, APC-Cy7-anti-CD8, and FITC-anti-CD69. (A) Expression of CD69 on the CD4+CD8+ DP thymocytes from control B6 mice (Cont), and mice 5 (5d) and 7 days (7d) after cyclophosphamide treatment. The histograms show expression of CD69 in gated 4-1BB (A) and 4-1BBL (B) positive CD4+CD8+, CD4loCD8lo, CD4+CD8lo, and CD4loCD8+ thymocytes from mice 5 days after cyclophosphamide treatment. Data are representative of three independent experiments with four or more animals in each group.
4-1BBL up-regulates expression of 4-1BB and 4-1BBL in mouse thymocytes
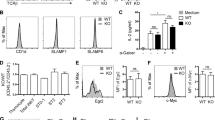
To investigate the function of 4-1BBL, we tested its ability to stimulate 4-1BB expression in mouse thymocytes. Flow cytometric analysis revealed greatly increased expression of 4-1BB on mouse thymocytes grown for 48 h after exposure to 100 ng/ml 4-1BBL, compared to control thymocytes cultured for 48 h and freshly isolated thymocytes; only slight up-regulation of 4-1BBL expression was observed 12 h after 4-1BBL treatment (Figures 8A and 8B). In contrast to 4-1BB expression, expression of 4-1BBL was significantly higher in control thymocytes cultured for 48 h than in freshly isolated thymocytes (Figures 8A and 8B).
Flow cytometric analysis of the expression of 4-1BB and 4-1BBL in the mouse thymocytes. Freshly isolated thymocytes were exposed to 4-1BBL (100 ng/ml), and incubated for 6, 12, 24 and 48 h. All the cells, except the freshly isolated thymocytes, were cultured in RPMI 1640 medium for the same length of time (48 h) regardless of the duration of treatment with 4-1BBL, and stained with biotin-anti-4-1BB or biotin-anti-4-1BBL followed by PE-streptavidin. The histograms show the fluorescence intensities of 4-1BB and 4-1BBL. Data are representative of three independent experiments with similar results.
4-1BBL enhances thymocyte adhesion to mouse thymic epithelial cells
To assess whether signaling through 4-1BB affects thymocyte adhesion to thymic epithelial cells we performed a cell adhesion assay. We first checked the expression of 4-1BB in mouse thymic epithelial cells by RT-PCR and Western blotting, and detected their expression of both 4-1BB mRNA and protein. Interestingly, it was found that a significant increase in the number of thymocytes adhering to the thymic epithelial cells after treatment with 4-1BBL (Figure 9), indicating that 4-1BBL evidently promotes thymocyte adhesion to thymic epithelial cells.
Quantitative assay of adhesion of thymocytes to the mouse thymic epithelial cells. Thymocytes were seeded onto cultured thymic epithelial cells, exposed to 4-1BBL (100 ng/ml) and incubated for 12 and 24 h. (A) RT-PCR analysis of 4-1BB and GAPDH mRNA in thymic epithelial cells. (B) Western blot analysis of 4-1BB and α-tubulin protein in thymic epithelial cells. Thymocytes adhering to the thymic epithelial cells before (C) and after (D) 4-1BBL treatment were photographed under a light microscope at ×400 magnification. (E) Numbers of adherent thymocytes determined by cell counting. Data are expressed as means + SD. ***P < 0.001, by t-test. Data are representative of more than three independent experiments with similar results.
Discussion
We have shown in the present study that 4-1BB and 4-1BBL are present on the thymocytes of normal and regenerating thymus, and that they are markedly up-regulated - predominantly in the CD4+CD8+ DP, and the CD4loCD8lo, CD4+CD8lo and CD4loCD8+ intermediate thymocyte subsets - during thymic regeneration after the acute thymic involution induced by cyclophosphamide treatment of mice.
The expression of 4-1BBL in the nine thymocyte subpopulations during thymus regeneration exhibited essentially the same pattern as that of 4-1BB, indicating that 4-1BB and 4-1BBL are expressed in the same compartment of the thymus associated with the developmental stages of the CD4+CD8+ DP, and the intermediate thymocyte subsets, where interactions between 4-1BB and 4-1BBL take place to maintain the normal physiological functions of the thymus. Expression of 4-1BB and 4-1BBL during thymus regeneration in the four major thymocyte subsets was essentially the same as their expression in the nine thymocyte subpopulations except for the 4-1BBL expression in CD8+ SP thymocytes during thymus regeneration. However, we were subsequently able to show that the discrepancy between the expression of 4-1BBL in the CD8+ SP thymocytes analyzed in the four major thymocyte subsets and that analyzed in the nine thymocyte subsets was due to the fact that the CD8+ SP thymocytes examined in the four thymocyte subsets contained 4-1BBL positive CD4loCD8+ thymocytes.
TCR expression was also strikingly enhanced in the CD4+CD8+ DP, and intermediate thymocyte subsets during thymic regeneration, indicating that the expression of 4-1BB and 4-1BBL during thymic regeneration is associated with thymocyte maturation. TCR complexes are not only vital for the functioning of mature T lymphocytes, but are also responsible for directing the maturation of immature T cell precursors in the thymus. It has been demonstrated that different TCR sequences are associated with different maturation pathways (Correia-Neves et al., 2001), and several lines of evidence point to two major sequences of CD4/CD8 T cell differentiation: from CD4+CD8+TCRloCD69- to CD4loCD8lo/+TCRintCD69+ and finally to CD4+CD8loTCRint/hiCD69+ (to mature CD4+CD8-TCRhi) or CD4loCD8lo/+TCRhiCD69+ (to mature CD4-CD8+TCRhi) (Lucas and Germain, 1996; Anderson et al., 1999). 4-1BB and 4-1BBL expression on developing T cells during thymus regeneration agreed with these sequences, suggesting that 4-1BB and 4-1BBL may be involved in the differentiation of the thymocytes.
Importantly we found that 4-1BB and 4-1BBL were preferentially expressed in the positively selected thymocytes among the CD4+CD8+ DP and the intermediate thymocytes, suggesting a potential role of 4-1BB and 4-1BBL in positive selection or in regulating certain activities of postselected thymocytes during the transition from CD4+CD8+ DP to intermediate DP thymocytes. Positive selection is a key step in T cell development because it leads to the survival of CD4+CD8+ DP thymocytes already pre-programmed to undergo differentiation, and also provides essential differentiation-inducing signals (Anderson et al., 1997, 1999). It is well documented that TCR-mediated signals induce many phenotypic changes during the positive selection, including up-regulation of TCR expression on DP thymocytes, termination of the activities of the recombination-activating genes (RAG-1 and RAG-2), expression of CD69, and down-regulation of either CD4 or CD8 (Turka et al., 1991; Borgulya et al., 1992; Brändle et al., 1992, 1994; Bendelac et al., 1992; Swat et al., 1993; Yamashita et al., 1993; Anderson et al., 1997, 1999; Correia-Neves et al., 2001). In agreement with the two-signal hypothesis for T cell activation it has been shown that interactions between the TCR and MHC-peptide complexes are not enough to induce complete maturation of CD4+CD8+ DP thymocytes, suggesting that additional accessory signals are required (Groves et al., 1997; Anderson et al., 1999). In particular, the costimulatory molecules that participate in the initial phases of positive selection have not yet been identified, although some molecules expressed by CD4+CD8+ DP thymocytes, such as CD2, CD5, CD24, CD28, CD49d, CD81 and thymic shared antigen 1 (TSA-1), have been shown to possess costimulatory activity in vitro in the absence of thymic epithelium, suggesting that these accessory molecules are involved in positive selection (Cibotti et al., 1997; Anderson et al., 1999).
Furthermore, there is growing evidence for a role of 4-1BB and 4-1BBL in hematopoiesis. 4-1BBL is expressed on hematopoietic stem cells, differentiating common myeloid progenitors and granulocyte-macrophage progenitors; in addition, 4-1BB is inducible on activated myeloid progenitors, and both components are implicated in the development of DCs (Kim et al., 2002; Lee et al., 2008b). It has been also shown that 4-1BB and 4-1BBL are expressed by bone marrow and CD34 cells, respectively, and that reverse signaling through 4-1BBL enhances the proliferation of CD34+ cells and their differentiation to myeloid cells, especially macrophages (Jiang et al., 2008a, 2008b). Mice exposed to anti-4-1BB mAb displayed evidence of dysregulated hemopoiesis, and developed lymphopenia, thrombocytopenia, and anemia (Niu et al., 2007). These data point to a novel function of 4-1BB and 4-1BBL in the growth and differentiation of hematopoietic progenitor cells.
It is well known that thymocyte adhesion to thymic epithelial cells - i.e., the interaction between thymocytes and to thymic epithelial cells - constitutes key events in T cell development. In this context, our data that 4-1BBL promoted thymocyte adhesion to thymic epithelial cells suggest that 4-1BBL and 4-1BB play a role in the postnatal T cell development. Previous studies have shown that cell interactions between thymocytes and thymic epithelial cells through cell adhesion molecules such as ICAM-1 and VCAM-1 are indispensable for immature thymocytes to develop into mature T cells in the thymus (Fine and Kruisbeek, 1991; Salomon et al., 1994, 1997; Wada et al., 1996). Interestingly, preliminary results of our ongoing studies show some evidence that 4-1BBL upregulates the expression of ICAM-1 and VCAM-1 on the thymic epithelial cells, suggesting that 4-1BBL/4-1BB signaling in the thymic epithelial cells augments thymocyte-thymic epithelial cell interactions through stimulation of the expression of these adhesion molecules (unpublished results). However, the precise roles of 4-1BB and 4-1BBL in thymus function during thymus regeneration remain to be clarified.
Taken together, our findings suggest that 4-1BBL signaling via the 4-1BB receptor on thymocytes, especially the CD4+CD8+ DP and intermediate thymocyte subsets, is involved in the processes of positive selection and regeneration in the mouse thymus leading to the production of new functional T cells during thymic regeneration.
Methods
Cell lines and cell culture
The generation, maintenance, and functional characterization of the mouse thymic subcapsular cortex or thymic nurse epithelial cells (427.1) have been described by Faas et al. (1993). These cells were cultured in DMEM (Gibco BRL, Grand Island, NY) containing 10% FBS (Gibco BRL) and 2 mM glutamine (Sigma, St. Louis, MO).
Experimental model of acute thymic involution and regeneration
Adult male, specific pathogen-free, C57BL/6 mice were purchased from Dae Han Bio Link (Seoul, Korea). They were housed three to four per cage and maintained under a 12 h light/dark cycle at 24℃ in a specific pathogen-free and humidity-controlled facility and were provided with standard sterile food and water ad libitum. They were allowed to adjust to their environment for 1 week, and were used at 8-10 weeks of age. Since cyclophosphamide, a DNA alkylating agent commonly used in chemotherapy, is a long-established method of investigating thymic regeneration (Milićević et al., 1984; Yoon et al., 1997, 2003; Lee et al., 2005, 2007, 2008a), the animals were given a single intraperitoneal dose of cyclophosphamide (400 mg/kg body weight, Sigma) in normal saline, and were killed in groups of four or more at 1, 2, 3, 4, 5, 6, 7 and 10 days after injection. Mice given the same amount of normal saline were used as controls. Animal care and all experimental procedures were conducted in accordance with the "Guide for Animal Experiments" produced by the Korean Academy of Medical Sciences.
Antibodies and reagents
The following fluorochrome-conjugated mAbs were purchased from BD Biosciences (San Jose, CA): Pacific Blue-conjugated anti-CD4 (RM4-5), allophycocyanin (APC)-Cy7-conjugated anti-CD8 (53-6.7), fluorescein isothiocyanate (FITC)-conjugated anti-TCRβ (H57-597), and FITC-anti-CD69 (H1.2F3). Biotinylated anti-4-1BB and 4-1BBL antibodies were purchased from BioLegend (San Diego, CA) and goat polyclonal anti-4-1BB and anti-4-1BBL antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PE-streptavidin was from BD Biosciences and the 2.4G2 hybridoma (anti-FcγRII/III) was from the American Type Culture Collection (ATCC; Manassas, VA). Recombinant murine 4-1BBL was obtained from Peprotech (Rocky Hill, NJ).
Thymocyte isolation and flow cytometry
Immunophenotypic analysis of cells was performed by two-, three- or four-color analysis on a FACSCanto II (BD Biosciences) with FACSDiva software (BD Biosciences). Single cell suspensions were prepared from the thymus by pressing the thymus in a 70-µm nylon mesh cell strainer (BD Falcon, BD Biosciences), and depleted of red blood cells in ACK lysis solution. The number of cells isolated from the thymus was determined by counting live cells detected by trypan blue exclusion.
For flow cytometry, cells were first incubated with 2.4G2 culture supernatant to block nonspecific antibody binding and then stained with a combination of the indicated fluorochrome-conjugated or biotinylated mAbs. When biotinylated-mAbs were used, the cells were subsequently incubated with PE-streptavidin. Background fluorescence was determined on cells stained with fluorochrome-labeled isotype-matched nonreactive mAbs. An electronic gate was set on the thymocytes using forward and side scatter characteristics. In all experiments, cells were gated on forward and side scatter to eliminate dead/dying cells and debris.
Quantitation of thymocyte adhesion to thymic epithelial cells
The assay of adhesion of thymocytes to thymic epithelial cells was based on that described by Barda-Saad et al. (1996). Briefly, the mouse thymic epithelial cells were seeded in 60 mm culture dishes at a density of 3 × 105 cells per dish in DMEM containing 10% FBS, and incubated for 12 and 24 h after treatment with 4-1BBL (100 ng/ml). Freshly isolated thymocytes were seeded onto layers of the thymic epithelial cells at a density of 8 × 106 cells per dish, and incubated for 3 h. After removing non-adherent thymocytes by gentle washing, the adherent thymocytes were examined with an Olympus BX50 microscope, and photomicrographs were captured digitally at 1,360 × 1,024 pixel resolution with an Olympus DP70 digital camera. In addition, the adherent thymocytes were collected by gentle pipetting and counted using a hemocytometer after trypan blue staining.
Western blot analysis
Thymic proteins were isolated using a protein extraction solution (PRO-PREP Protein Extraction Solution, Intron, Seoul, Korea). The lysates were centrifuged at 13,000 rpm for 15 min at 4℃. Protein concentrations were determined with the Bradford protein assay reagent (Bio-Rad, Hercules, CA). Equal amounts of protein samples were heated for 10 min at 95℃ in sample buffer and separated by 10% SDS-PAGE, using a Mini-Protean III system (Bio-Rad, Melville, NY). The proteins were transferred onto a PVDF membrane (Bio-Rad) by semi-dry transfer (Bio-Rad), and the membrane was incubated overnight at 4℃ with anti-4-1BB (sc-11811, Santa Cruz Biotechnology) and anti-4-1BBL (sc-11819, Santa Cruz Biotechnology) at a dilution of 1:200 in Tris-buffered saline (TBS, 20 mM Tris-HCl, 150 mM NaCl, pH 7.4) containing 2% skim milk. After three washes with TBS-T (TBS containing 0.1% Tween 20) containing 1% skim milk, the membrane was incubated for 2 h at room temperature with secondary antibody (anti-goat IgG peroxidase conjugate, sc-2768, Santa Cruz Biotechnology) diluted 1:1000, and washed three times with TBS-T. Immunoreactivity was detected by enhanced chemiluminescence (ECL, SuperSignal West Pico Chemiluminescent Substrate kit, Pierce, Rockford, IL) according to the manufacturer's instructions, and images were captured and quantified with a LAS-3000 imaging system (Fujifilm, Tokyo, Japan). Data were expressed as ratios of 4-1BB and 4-1BBL normalized to α-tubulin to correct for any error in spectrophotometric protein quantification or in pipetting.
RT-PCR analysis
Total RNA was isolated using TRIzol® Reagent (Invitrogen, Carlsbad, CA); briefly, samples were transferred to tubes containing 1 ml of the RNA extraction solution. The homogenates were chloroform extracted, isopropanol precipitated, ethanol washed, and resuspended in 30 µl of distilled water. RNA concentrations and purity were determined by absorbance at 260 and 280 nm. Samples exhibiting an absorbance ratio (260/280) greater than or equal to 1.7 were used. First strand cDNA was obtained by reverse transcription (RT) using 2 µg of mouse thymocyte RNA. The reaction was conducted in 25 µl of buffer containing 0.5 µg of oligo (dT)12-18 primer (Promega), 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 40 mM DTT, 0.5 mM deoxynucleotide triphosphate (dNTP) mixture (Promega), 10 units RNase inhibitor (Promega), and 200 units of MMLV reverse transcriptase (Promega). After incubation at 42℃ for 60 min, the reaction was stopped by heating at 70℃ for 15 min and the cDNA was used as a template for PCR amplification using gene-specific primers for mouse 4-1BB and 4-1BBL. Specific primers were designed for each gene (Bioneer, Cheongwon-Kun, Chungbuk, Korea), and the primers for mouse 4-1BB consisted of forward primer (5'-GGGAAACAACTGTTACCACG-3'), corresponding to nucleotides 115-134, and reverse primer (5'-AGACCTTCCGTCTAGAGAGC-3') complementary to nucleotides 547-566 in the mouse 4-1BB gene sequence [GenBank:NM_011612]. The primers for mouse 4-1BBL consisted of forward primer (5'-CTCTCCTGTGTTCGCCAAGC-3'), corresponding to nucleotides 399-418, and reverse primer (5'-CCAGCCTTCAGGAGCAACAG-3') complementary to nucleotides 825-844 in the mouse 4-1BBL gene sequence [GenBank:NM_009404]. These were used to amplify 452 bp and 366 bp fragments, respectively, and to detect the mouse 4-1BB and 4-1BBL transcripts. The cDNA was amplified with an automated thermal cycler (TECHNE, Teddington, UK) in a final volume of 25 µl containing 2 µl of cDNA solution, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 0.1% Triton X-100, 0.2 mM dNTP mixture (Promega), 0.4 pmol of each primer and 5 units of Taq DNA polymerase (Promega). The amplification procedure consisted of an initial denaturation at 94℃ for 5 min followed by 28 cycles of denaturation at 94℃ for 30 s, primer annealing at 57℃ for 30 s, and extension at 72℃ for 30 s, with a final extension at 72℃ for 10 min, and ending with a 4℃ hold cycle. The amplified products were analyzed by electrophoresis on 1.5% agarose gels, stained with ethidium bromide and visualized under UV light. Band intensities of the PCR products were measured using an image analysis program (MetaMorph, Universal Imaging Corporation, Downingtown, PA). Data were expressed as ratios of 4-1BB and 4-1BBL mRNA normalized to GAPDH mRNA amplified from the same cDNA samples.
Statistical analysis
Data are expressed as means + SD. For comparisons of multiple groups, we used one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. In addition, to compare pairs of groups we used Student's two-tailed t-test. Statistical significance was set at P < 0.05.
Abbreviations
- ATCC:
-
American type culture collection
- DC:
-
dendritic cells
- DN:
-
double-negative
- dNTP:
-
deoxynucleotide triphosphate
- DP:
-
double-positive
- SP:
-
single-positive
- NGFR:
-
nerve growth factor receptor
- RAG:
-
recombination-activating gene
- TBS:
-
Tris-buffered saline
- TNFR:
-
tumor necrosis factor receptor
- TSA-1:
-
thymic shared antigen-1
- FITC:
-
fluorescein isothiocyanate
References
Anderson G, Hare KJ, Platt N, Jenkinson EJ . Discrimination between maintenance- and differentiation-inducing signals during initial and intermediate stages of positive selection . Eur J Immunol 1997 ; 27 : 1838 - 1842
Anderson G, Hare KJ, Jenkinson EJ . Positive selection of thymocytes: the long and winding road . Immunol Today 1999 ; 20 : 463 - 468
Barda-Saad M, Rozenszajn LA, Globerson A, Zhang AS, Zipori D . Selective adhesion of immature thymocytes to bone marrow stromal cells: relevance to T cell lymphopoiesis . Exp Hematol 1996 ; 24 : 386 - 391
Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH . Activation events during thymic selection . J Exp Med 1992 ; 175 : 731 - 742
Bertram EM, Lau P, Watts TH . Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection . J Immunol 2002 ; 168 : 3777 - 3785
Borgulya P, Kishi H, Uematsu Y, von Boehmer H . Exclusion and inclusion of α and β T cell receptor alleles . Cell 1992 ; 69 : 529 - 537
Brändle D, Müller C, Rülicke T, Hengartner H, Pircher H . Engagement of the T-cell receptor during positive selection in the thymus down-regulates RAG-1 expression . Proc Natl Acad Sci USA 1992 ; 89 : 9529 - 9533
Brändle D, Müller S, Müller C, Hengartner H, Pircher H . Regulation of RAG-1 and CD69 expression in the thymus during positive and negative selection . Eur J Immunol 1994 ; 24 : 145 - 151
Cho HR, Kwon B, Yagita H, La S, Lee EA, Kim JE, Akiba H, Kim J, Suh JH, Vinay DS, Ju SA, Kim BS, Mittler RS, Okumura K, Kwon BS . Blockade of 4-1BB (CD137)/4-1BB ligand interactions increases allograft survival . Transpl Int 2004 ; 17 : 351 - 361
Cibotti R, Punt JA, Dash KS, Sharrow SO, Singer A . Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals . Immunity 1997 ; 6 : 245 - 255
Correia-Neves M, Mathis D, Benoist C . A molecular chart of thymocyte positive selection . Eur J Immunol 2001 ; 31 : 2583 - 2592
Croft M . Co-stimulatory members of the TNFR family: keys to effective T-cell immunity ? Nat Rev Immunol 2003 ; 3 : 609 - 620
Faas SJ, Rothstein JL, Kreider BL, Rovera G, Knowles BB . Phenotypically diverse mouse thymic stromal cell lines which induce proliferation and differentiation of hematopoietic cells . Eur J Immunol 1993 ; 23 : 1201 - 1214
Fine JS, Kruisbeek AM . The role of LFA-1/ICAM-1 interactions during murine T lymphocyte development . J Immunol 1991 ; 147 : 2852 - 2859
Foell J, Strahotin S, O'Neil SP, McCausland MM, Suwyn C, Haber M, Chander PN, Bapat AS, Yan XJ, Chiorazzi N, Hoffmann MK, Mittler RS . CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB × NZW F1 mice . J Clin Invest 2003 ; 111 : 1505 - 1518
Goodwin RG, Din WS, Davis-Smith T, Anderson DM, Gimpel SD, Sato TA, Maliszewski CR, Brannan CI, Copeland NG, Jenkins NA, Farrah T, Armitage RJ, Fanslow WC, Smith CA . Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor . Eur J Immunol 1993 ; 23 : 2631 - 2641
Groves T, Parsons M, Miyamoto NG, Guidos CJ . TCR engagement of CD4+CD8+ thymocytes in vitro induces early aspects of positive selection, but not apoptosis . J Immunol 1997 ; 158 : 65 - 75
Halstead ES, Mueller YM, Altman JD, Katsikis PD . In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses . Nat Immunol 2002 ; 3 : 536 - 541
Hurtado JC, Kim YJ, Kwon BS . Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death . J Immunol 1997 ; 158 : 2600 - 2609
Jiang D, Chen Y, Schwarz H . CD137 induces proliferation of murine hematopoietic progenitor cells and differentiation to macrophages . J Immunol 2008a ; 181 : 3923 - 3932
Jiang D, Yue PS, Drenkard D, Schwarz H . Induction of proliferation and monocytic differentiation of human CD34+ cells by CD137 ligand signaling . Stem Cells 2008b ; 26 : 2372 - 2381
Kim YJ, Li G, Broxmeyer HE . 4-1BB ligand stimulation enhances myeloid dendritic cell maturation from human umbilical cord blood CD34+ progenitor cells . J Hematother Stem Cell Res 2002 ; 11 : 895 - 903
Kwon BS, Weissman SM . cDNA sequences of two inducible T-cell genes . Proc Natl Acad Sci USA 1989 ; 86 : 1963 - 1967
Laderach D, Wesa A, Galy A . 4-1BB-ligand is regulated on human dendritic cells and induces the production of IL-12 . Cell Immunol 2003 ; 226 : 37 - 44
Lee HW, Kim BS, Kim HJ, Lee CW, Yoo HJ, Kim JB, Yoon S . Upregulation of receptor activator of nuclear factor-κB ligand expression in the thymic subcapsular, paraseptal, perivascular, and medullary epithelial cells during thymus regeneration . Histochem Cell Biol 2005 ; 123 : 491 - 500
Lee HW, Kim SM, Shim NR, Bae SK, Jung IG, Kwak JY, Kim BS, Kim JB, Moon JO, Chung JS, Yoon S . Expression of nerve growth factor is upregulated in the rat thymic epithelial cells during thymus regeneration following acute thymic involution . Regul Pept 2007 ; 141 : 86 - 95
Lee HW, Park HK, Na YJ, Kim CD, Lee JH, Kim BS, Kim JB, Lee CW, Moon JO, Yoon S . RANKL stimulates proliferation, adhesion and IL-7 expression of thymic epithelial cells . Exp Mol Med 2008a ; 40 : 59 - 70
Lee SW, Park Y, So T, Kwon BS, Cheroutre H, Mittler RS, Croft M . Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells . Nat Immunol 2008b ; 9 : 917 - 926
Lenschow DJ, Walunas TL, Bluestone JA . CD28/B7 system of T cell costimulation . Annu Rev Immunol 1996 ; 14 : 233 - 258
Lippert U, Zachmann K, Ferrari DM, Schwarz H, Brunner E, Mahbub-Ul Latif AH, Neumann C, Soruri A . CD137 ligand reverse signaling has multiple functions in human dendritic cells during an adaptive immune response . Eur J Immunol 2008 ; 38 : 1024 - 1032
Lucas B, Germain RN . Unexpectedly complex regulation of CD4CD8 coreceptor expression supports a revised model for CD4+/CD8+ thymocyte differentiation . Immunity 1996 ; 5 : 461 - 477
May KF, Chen L, Zheng P, Liu Y . Anti-4-1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells . Cancer Res 2002 ; 62 : 3459 - 3465
Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, Mittler RS, Chen L . Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors . Nat Med 1997 ; 3 : 682 - 685
Milićević NM, Milićević Ž, Piletić O, Mujović S, Ninkov V . Patterns of thymic regeneration in rats after single or divided doses of cyclophosphamide . J Comp Pathol 1984 ; 94 : 197 - 202
Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK . Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy . J Exp Med 1999 ; 190 : 1535 - 1540
Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, Spencer T, Dillehay D, Kwon B, Chen L, Vella AT, Mittler RS . Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice . J Immunol 2007 ; 178 : 4194 - 4213
Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS . Inducible T cell antigen 4-1BB. Analysis of expression and function . J Immunol 1993 ; 150 : 771 - 781
Salomon DR, Mojcik CF, Chang AC, Wadsworth S, Adams DH, Coligan JE, Shevach EM . Constitutive activation of integrin α4β1 defines a unique stage of human thymocyte development . J Exp Med 1994 ; 179 : 1573 - 1584
Salomon DR, Crisa L, Mojcik CF, Ishii JK, Klier G, Shevach EM . Vascular cell adhesion molecule-1 is expressed by cortical thymic epithelial cells and mediates thymocyte adhesion. Implications for the function of α4β1 (VLA4) integrin in T-cell development . Blood 1997 ; 89 : 2461 - 2471
Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, Bangia N, DeBenedette MA, Mak TW, Choi Y, Watts TH . CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand . J Exp Med 1998 ; 187 : 1849 - 1862
Schwarz H . Biological activities of reverse signal transduction through CD137 ligand . J Leukoc Biol 2005 ; 77 : 281 - 286
Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS . 4-1BB-mediated immunotherapy of rheumatoid arthritis . Nat Med 2004 ; 10 : 1088 - 1094
Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS . 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses . J Exp Med 1997 ; 186 : 47 - 55
Sica G, Chen L . Modulation of the immune response through 4-1BB . Adv Exp Med Biol 2000 ; 465 : 355 - 362
Sun Y, Chen HM, Subudhi SK, Chen J, Koka R, Chen L, Fu YX . Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease . Nat Med 2002a ; 8 : 1405 - 1413
Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu YX . Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis . J Immunol 2002b ; 168 : 1457 - 1465
Swat W, Dessing M, von Boehmer H, Kisielow P . CD69 expression during selection and maturation of CD4+8+ thymocytes . Eur J Immunol 1993 ; 23 : 739 - 746
Takahashi C, Mittler RS, Vella AT . Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal . J Immunol 1999 ; 162 : 5037 - 5040
Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP . 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses . J Immunol 1999 ; 163 : 4859 - 4868
Turka LA, Schatz DG, Oettinger MA, Chun JJ, Gorka C, Lee K, McCormack WT, Thompson CB . Thymocyte expression of RAG-1 and RAG-2: termination by T cell receptor crosslinking . Science 1991 ; 253 : 778 - 781
Wada K, Kina T, Kawamoto H, Kondo M, Katsura Y . Requirement of cell interactions through adhesion molecules in the early phase of T cell development . Cell Immunol 1996 ; 170 : 11 - 19
Watts TH . TNF/TNFR family members in costimulation of T cell responses . Annu Rev Immunol 2005 ; 23 : 23 - 68
Yamashita I, Nagata T, Tada T, Nakayama T . CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor-mediated positive selection . Int Immunol 1993 ; 5 : 1139 - 1150
Ye Z, Hellström I, Hayden-Ledbetter M, Dahlin A, Ledbetter JA, Hellström KE . Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB . Nat Med 2002 ; 8 : 343 - 348
Yoon S, Yoo YH, Kim BS, Kim JJ . Ultrastructural alterations of the cortical epithelial cells of the rat thymus after cyclophosphamide treatment . Histol Histopathol 1997 ; 12 : 401 - 413
Yoon S, Lee HW, Baek SY, Kim BS, Kim JB, Lee SA . Upregulation of TrkA neurotrophin receptor expression in the thymic subcapsular, paraseptal, perivascular, and cortical epithelial cells during thymus regeneration . Histochem Cell Biol 2003 ; 119 : 55 - 68
Acknowledgements
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006-311-E00150) and by the MRC program of MOST/KOSEF (R13-2005-009). Seong-A Ju was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-412-J00302).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kim, YM., Kim, H., Kim, H. et al. Expression of 4-1BB and 4-1BBL in thymocytes during thymus regeneration. Exp Mol Med 41, 896–911 (2009). https://doi.org/10.3858/emm.2009.41.12.095
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2009.41.12.095
Keywords
This article is cited by
-
Generation of T-cell-receptor-negative CD8αβ-positive CAR T cells from T-cell-derived induced pluripotent stem cells
Nature Biomedical Engineering (2022)
-
Absence of Grail promotes CD8+ T cell anti-tumour activity
Nature Communications (2017)