Abstract
Atopic dermatitis (AD) is an inflammatory skin disorder that is both uncomfortable and distressing to patients, and its prevalence has been steadily increasing. It is obvious that the identification of efficient markers of AD in plasma would offer the possibility of effective diagnosis, prevention, and treatment strategies. In this study, a proteomic approach was used to analyze plasma glycoproteins from both children with AD and healthy child donors. Several protein spots showing significant quantitative changes in the AD patients were identified. Through sequential studies, it was confirmed that CD5L and ApoE were significantly up-regulated or down-regulated, respectively, in the plasma from AD patients compared with that from healthy donors. In addition, we suggest that the up-regulated CD5L in AD patients causes eosinophilia by inhibiting apoptosis or promoting the proliferation of eosinophils either in combination with or without IL-5. The glycoproteomic data in this study provides clues to understanding the mechanism of atopic alterations in plasma and suggests AD-related proteins can be used as candidate markers for AD.
Similar content being viewed by others
Introduction
Atopic dermatitis (AD) is a common pruritic and chronically relapsing inflammatory skin disease. AD commonly occurs in infancy and early childhood, but can also start or persist in adulthood (Abramotiv, 2005; Scirica et al., 2007). The pathophysiology of AD includes a disturbed skin barrier function, frequent allergic responses to allergens, defects in the antimicrobial immune defense, and a genetic predisposition. The number of AD patients has increased with modernization and industrialization; particularly, a dramatic increase of the incidence of AD has occurred in industrialized countries (Gold and Kemp, 2005). To develop effective therapeutic strategies and monitor the progress of an AD patient, a deeper understanding of the complex pathogenesis of AD is necessary.
Proteomic approaches have been applied to analyze many diseases and to probe for therapeutic purposes (Yuk and Ha, 2005; Lee et al., 2006; Kang et al., 2007; Lovestone et al., 2007). Among various subproteomes, the plasma proteome represents comprehensive physiological or pathological states. Thus, analyzing the plasma proteome can provide valuable information for mining disease marker proteins. Glycoproteome is also a major subproteome: many clinical biomarkers and therapeutic targets are glycoproteins. In particular, the glycosylation patterns are influenced considerably by the physiological status (Kim et al., 2004; Zhao et al., 2006). Therefore, in this study, a differential glycoprotein profiling of plasma trials of healthy donors and AD patients was performed. Compared with the healthy donors, several differentially expressed proteins were identified in the results of AD patients. Among them, it was found that CD5L and ApoE are potential biomarkers in the diagnosis of AD or in tracking its progress.
Materials and Methods
Reagents
The anti-CD5L antibody (AF2797) and recombinant CD5L protein (2797-CL, MN) were procured from R&D Systems (Minneapolis, MN). The anti-ApoE antibody was supplied by Santa Cruz Biotechnology (Santa Cruz, CA). The urea, DTT, immobilized pH gradient (IPG) buffer, and the drystrip cover fluid were obtained from GE HealthCare (Uppsala, Sweden). The CHAPS, Tris, glycine, iodoacetamide, bromophenol blue, thiourea, and ammonium bicarbonate were provided by Sigma (St. Louis, MO).
Subjects
Human plasma samples were taken from healthy donors and patients with AD. The age of both the control and AD patients ranged from 2 to 7 years old. The protocols of this study were approved by the Institutional Review Board (IRB) of KRIBB with the informed consent of the healthy donors and AD patients. Diagnosis of AD was based on the criteria proposed by Hanifin and Rajka (Hanifin and Rajka, 1980) for the criteria to assess the severity of AD, SCORAD (SCORing Atopic Dermatitis) was used (Table 1). The plasma parameters were measured in a clinical laboratory, and all clinical data are summarized in Table 1. The control group consisted of eight children of similar age without a personal or family history of atopic diseases.
Purification of glycoproteins from plasma
To selectively isolate the glycoproteins from the plasma, a Qproteome total glycoprotein kit (Qia-37551, Qiagen, Valencia, CA) was used. First, the spin column was washed with 2.5 ml binding buffer containing 20 µl of protease inhibitor solution. Then, 50 µl of the plasma sample diluted with 500 µl of binding buffer was loaded onto the column and incubated. Next, the column was centrifuged for 2 min at 500 rpm to remove the nonbonding fraction. After that, the column was washed with 750 µl of binding buffer twice to wash off the nonspecific binding. The captured glycoproteins were eluted with 100 µl of elution buffer ME twice and then with 100 µl of elution buffer SE three times. The eluted fraction was collected via centrifugation at 500 rpm for 2 min. The eluted fractions were pooled and a DC protein assay kit (BioRad, Hercules) was used to monitor the protein concentrations.
2-DE and image analysis
The samples were applied to precast IPG strips with a nonlinear pH range of 4-7. Samples were applied via the rehydration of the IPG strips in a sample solution (8 M Urea, 2% Thiourea, 4% CHAPS, 1 mM DTT, 0.5% IPG buffer, a trace amount of bromophenol blue, and 50 mM Tris-HCl, pH 7.5) for more than 12 h and run for a first-dimensional IEF. After the IEF, the IPG strips were equilibrated twice for 15 min. The first equilibrium solution contained 50 mM of pH 8.8 Tris-HCl buffer with 6 M urea, 20% glycerol, 2% SDS, and 1% DTT. In the second equilibrium solution, the DTT was replaced with 2.5% iodoacetamide. The proteins in the equilibrated strips were separated using a second-dimensional 12% SDS-PAGE. After electrophoresis, the protein spots were visualized using Coomassie staining. The images were then processed with Phoretix 2-DE expression software (Nonlinear Dynamics Ltd., UK). The spot volumes were normalized based on the total spot volume of each gel. The protein spot intensity was defined as the normalized spot volume that is the ratio of the single spot volume to the total spot volume on a 2-D gel (total spot normalization). Computer analysis allowed the automatic detection and quantification of the protein spots as well as matching between the control gels and gels from AD samples. Statistical significance was accepted at a level of P < 0.05.
In-gel digestion and mass spectrometry
The spots of interest were manually excised. The excised protein spots were digested using trypsin for 16-24 h at 37℃. The digested peptides were extracted via sonication for 25 min with an extraction solution [50% acetonitrile (ACN) and 2.5% trifluoroacetic acid (TFA)], and the extracted peptides were dried in a vacuum. Then, the extracted peptides were resuspended in 10 µl 0.1% TFA followed by desalting/concentration with µZipTipC18 (Millipore Co. Bedford, MA). For peptide mass fingerprinting, the desalted peptides were dissolved with 1 µl of 50% ACN and 0.1% TFA, and a 0.5 µl drop of peptide was applied to the target plate and allowed to dry in air. The mass spectra were acquired via an Axima CFR+ matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS, Shimadzu Biotech, Japan). The obtained mass spectra were analyzed using a MASCOT PMF search. The following parameters were used: the database was NCBInr (species: Homo sapiens) with the maximum number of missed cleavage by trypsin at 1. The mass tolerance ranged from ± 50 to ± 100 ppm. The allowed peptide modification was carbamidomethylation in a fixed modification mode. The proteins that failed by peptide mass fingerprinting were tried again using LC-MS/MS (Q-TOF Premier, Micromass, UK).
Western blot analysis
The samples were separated using SDS-PAGE. The protein was transferred onto a PVDF membrane and blotted with the primary antibody using standard methods. Detection was achieved using ECL plus Western blotting reagents (Pierce). The bands were scanned with a flat-bed scanner and analyzed using image analysis software.
Cell viability assay of AML14.3D10 cells
AML 14.3D10 cells were maintained in a RPMI 1640 medium containing 10% v/v FBS (Gibco, Carlsbad, CA) and 1% v/v Antibiotic-Antimycotic (Gibco, Carlsbad, CA). Approximately 1 × 106 AML14.3D10 cells were resuspended in 20 ml of fresh complete medium and incubated for 24 h. Then, the cells were treated with IL-5 (final 50 ng/ml) or dexamethasone (final 10 µM) in combination with various concentrations of CD5L for 24 h at 37℃ in a 5% CO2 incubator. Before harvesting, the cultured cells were washed twice with ice-cold PBS. The cell viability was assessed by measuring the ability of the cells to MTT, as previously described in the literature (Kang et al., 2007; Lee et al., 2007).
Results and Discussion
Clinical data in plasma samples of AD patients
AD is very common in children, typically before the age of 5 (Scirica et al., 2007). Therefore, the focus of this investigation is on AD in young children. The diagnosis and severity of AD were monitored according to the SCORAD value of the patients. SCORAD was proposed to standardize the clinical evaluation of AD patients; it is now widely used as a clinical tool for assessing the severity of AD. The SCORAD index values of the AD patients in this study were between 21 and 80. The majority of patients (6 of 8 patients) had a SCORAD index above 31. Therefore, the clinical picture was moderately severe. All clinical data, including the SCORAD values, are summarized in Table 1.
Isolation of glycoproteins from plasma of healthy donors and AD patients
Protein glycosylation plays a vital role in a wide range of cellular processes, such as cell adhesion and signaling, stabilization of protein folding and function, oncogenesis, and immune responses (Hakomori, 1989; Kim and Varki, 1997). It is proposed that nearly 50% of human plasma proteins are glycosylated. To test the possibility that the expression or glycosylation pattern of plasma glycoproteins are altered or changed in AD patients, Lens culinaris agglutinin (LCA) lectin blot analyses of the plasma proteins obtained from healthy donors and AD patients were initially conducted. LCA binds α1,6-fucose branching with a weak affinity, thus showing a relatively broad spectrum of carbohydrate moieties for recognition. Figure 1 shows the representative results of a 2-DE LCA lectin blot. It is noteworthy that the plasma from the AD patients shows a somewhat different pattern compared with that from healthy donors. This result strongly suggests that differential expressions or glycosylation patterns of several glycoproteins may be related to AD pathogenesis. Next, to thoroughly investigate the differential expression profiling of glycoproteins from the plasma of AD patients, analysis of the plasma glycoproteins was undertaken using proteomic tools. First, an LCA-sepharose 4B column was used to isolate the glycoproteins from the human plasma samples. However, this column did not efficiently remove most of the serum albumin; consequently, the presence of various low-abundant proteins on the 2-DE gel was masked (data not shown). It was then decided that a Qproteome total glycoprotein kit would be used to efficiently enrich the plasma glycoproteins. The total glycoprotein spin columns in the Qproteome total glycoprotein kit contained ConA and WGA lectins. They were used for the general enrichment of the total glycoprotein population from the plasma/serum samples. 1-DE gel analysis of the glycoprotein fractions shows efficient removal of albumin and a similar overall glycoprotein pattern of the plasma from the healthy donors and AD patients (data not shown).
Representative LCA lectin blot analysis of plasma from healthy control and AD patient. Total plasma proteins extracted from the healthy donors and the AD patients were separated on 2-DE gels and transferred onto a PVDF membrane. The membrane was subjected to a lectin blot and treated with biotin-labeled LCA. Spots that displayed differently (marked by circles) were detected.
Differentially expressed glycoprotein spots in plasma of patients with AD
To investigate the glycoproteins that showed significant quantitative changes in the plasma between the healthy donors and the AD patients, the plasma glycoproteins enriched by a Qproteome total glycoprotein kit were separated by 2-DE (Figure 2A). The protein spots that showed significant changes, greater than 1.5 fold in magnitude, between the healthy donors and the AD patients were scored. Statistical significance was accepted at a level of P < 0.05. A total of 29 spots showed a significant change in the AD patients. Among these, a total of 16 spots were identified using MALDI-TOF or ESI-Q TOF MS/MS. These are listed in Table 2. Of these, six spots including CD5L were up-regulated and ten spots including ApoE were down-regulated (Figures 2B and C, respectively). The glycoproteome results demonstrated that various proteins involved in blood clotting (coagulation), including fibrinβ, fibrinogenγ, C4B4, and IGHA1 protein, were decreased in the plasma of the AD patients.
The analysis of AD plasma using 2-DE. (A) Representative 2-DE map of plasma from an AD patient. The differentially expressed protein spots are indicated by a circle or rectangle. The results are summary of 2-DE results from all AD patients and normal control. The spots indicated were identified using either MALDI-TOF or LC-MS/MS, as outlined in Table 2. (B) Enlargement of the 2-DE gel on up-regulated and down-regulated spots. (C) Quantitation of the proteins shown in (B). The control volume is expressed as 100% and the volumes of the AD patients as values relative to the control. The data represent the mean values ± SD. *P < 0.05; **P < 0.01.
Validation of ApoE protein level in AD patients
To verify the differential expression of the ApoE protein, the plasma expression level of ApoE was also analyzed via Western blotting. As shown in Figure 3, the level of ApoE in the plasma from the AD patients was significantly reduced (P < 0.05). ApoE has long been known to be an essential constituent of plasma lipoproteins. In addition to its well-recognized roles in lipid metabolism and atherosclerogenesis, ApoE has been shown to be involved in several pathophysiologic processes not directly related to lipid transport. Recently, it was known that inflammation could impair lipoprotein metabolism and produce a wide variety of changes in the plasma concentrations of lipids and lipoproteins (Ripolles et al., 2006). In the glycoproteome data in this study, several apolipoproteins were differentially expressed. While ApoE was down-regulated, proapolipoprotein and the crystal structure of lipid-free human apolipoprotein AI were up-regulated. Therefore, it is speculated that the plasma lipoprotein level of the AD patients changes as a result of the inflammatory reaction during AD pathogenesis. Further studies regarding ApoE are necessary to provide insight into the pathogenesis of AD and whether ApoE can be utilized as a potential specific marker protein for AD.
Validation of the ApoE expression level via Western blotting. (A) Western blot analyses of serum protein (5 µl) from four healthy donors and six AD patients were conducted. (B) Quantitation analysis of the gel bands was conducted using Image J software. Significant differences (P < 0.05) were measured via a median plus ANOVA.
Validation of CD5L protein expression level in AD patients and the effect on the proliferation of eosinophils
CD5L (also known as an IgM-associated peptide, apoptosis inhibitor 6, and Spα) is a soluble protein belonging to group B of the scavenger receptor cysteine-rich (SPCR) superfamily (Sarrias et al., 2004, 2005). It is expressed through macrophages present in lymphoid tissues, and it binds to different cells of the immune system, which suggests that it may play a role in immune system regulation. In addition, CD5L appears to play a role as an inhibitor of apoptosis. It was demonstrated that CD5L supports macrophage survival and enhances the phagocytic function of macrophages in Corynebacterium parvum-induced hepatitis (Haruta et al., 2001). Recently, it was reported that CD5L inhibits the apoptosis of T cells and NKT cells in Corynebacterium-induced granuloma formation in mice (Kuwata et al., 2003). Yusa et al. also reported that CD5L induced strong and sustained growth inhibition of B lymphocytes in combination with TGF-β1 (Yusa et al., 1999). Therefore, it has been proposed that CD5L has different functions depending on the types of target cells and combination with other cytokines.
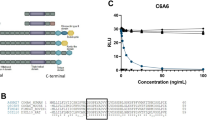
A Western blot analysis was conducted on the plasma from the AD patients using anti-CD5L antibody (Figure 4A). As shown, CD5L is significantly up-regulated in the plasma of the AD patients. Following this, we tested the effect of CD5L on the proliferation of the AML14.3D10 human eosinophil cell line (Figure 4B). The AML14.3D10 human myeloid leukemic cell line has been used as a model cell line for the study of eosinophil (Baumann and Paul, 1998; Yoon et al., 2005). Interestingly, CD5L increases the proliferation of the AML14.3D10 cell in a dose-dependent manner with IL-5 (Figure 4C). Furthermore, CD5L prevents AML14.3D10 cells from apoptosis or the growth inhibition effects induced by treatment with dexamethasone (Figure 4D). These data regarding the effect of CD5L on the proliferation of the eosinophil cell line strongly suggest that there is an interconnection between the CD5L plasma level and AD. Eosinophils act as effectors in the inflammatory reactions of allergic diseases including AD (Trautmann et al., 2001; Simon et al., 2004; Ryu et al., 2007). AD patients suffer from eosinophilia, an accumulation of eosinophils due to the increased survival or decreased apoptosis of eosinophils, which correlate with disease activity (Trautmann et al., 2000; Domae et al., 2003). It is known that the inhibition of the apoptosis of eosinophils can be achieved by an increased expression of survival factors, as well as a disruption of death signals (Simon, 2000). It is known that IL-5 is the most critical cytokine in mediating increased eosinophil differentiation, activation, and survival (Oldhoff et al., 2005; Simon and Simon, 2007). Based on these results and other reports, it is suggested that an increased CD5L may induce eosinophilia in AD patients in either in combination with or without IL-5 as a survival factor. Therefore, CD5L may be feasible as a potential biomarker in the diagnosis and treatment of AD, although CD5L was reported as candidate marker in asthma and liver cirrhosis (Wu et al., 2005; Gangadharan et al., 2007).
Validation of the CD5L expression level and effect of CD5L on AML14.3D10 proliferation. (A) Western blot analysis of the serum protein (5 µl) from six healthy donors and six AD patients were conducted. Quantitation analyses of the gel bands were conducted using Image J software. Significant differences (P < 0.01) were measured via a median plus ANOVA. (B) CD5L increases the proliferation of the AML14.3D10 cells. AML14.3D10 cells were seeded into 96 well plates and grown for 24 h. The cells were then treated with CD5L (± IL-5 or Dexamethasone). At each time point, the cell viability was measured. (C) CD5L induces the proliferation of AML14.3D10 cells in a dose-dependent manner in the presence of IL-5. (D) CD5L induces the proliferation of AML14.3D10 cells in a dose-dependent manner in the presence of Dexamethasone.
In conclusion, several glycoproteins that may be involved in AD were identified through a glycoproteomic approach, even though the number of patients was rather small. Among them, it was confirmed that CD5L and ApoE were differentially expressed in the plasma of AD patients. According to gene expression data from normal and AD skin tissue samples (http://www.ncbi.nlm.gov/geo/gds_browse.cgi?gds=2381), CD5L and ApoE are also differentially expressed in AD patients, which is consistent with our proteomic results. In future studies, we plan to investigate the detailed roles of the altered levels of these proteins during the progression of AD pathogenesis and whether or not these changes are specific to AD. This glycoproteomic analysis and further characterization of the identified proteins can provide valuable information that can build a deeper understanding of AD.
Abbreviations
- AD:
-
atopic dermatitis
- 2-DE:
-
two-dimensional electrophoresis
References
Abramotiv W . Atopic dermatitis . J Am Acad Dermatol 2005 ; 53 : S86 - S93
Baumann MA, Paul CC . The AML14 and AML14.3D10 cell lines: a long-overdue model for the study of eosinophils and more . Stem Cells 1998 ; 16 : 16 - 24
Domae M, Sagara H, Sakaue M, Fukuda T, Kamikawa Y . The antiallergic drug oxatomide promotes human eosinophil apoptosis and suppresses IL-5-induced eosinophil survival . J Allergy Clin Immunol 2003 ; 111 : 567 - 572
Gangadharan B, Antrobus R, Dwek RA, Zitzmann N . Novel serum biomarker candidates for liver fibrosis in hepatitis C patients . Clin Chem 2007 ; 53 : 1792 - 1799
Gold MS, Kemp AS . Atopic disease in childhood . Med J Aust 2005 ; 182 : 298 - 304
Hakomori S . Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens . Adv Cancer Res 1989 ; 52 : 257 - 331
Hanaffin JM, Rajka G . Diagnostic feature of atopic dermatitis . Acta Derm Venereol Suppl (Stockh) 1980 ; 92 : 44 - 47
Haruta I, Kato Y, Hashimoto E, Minjares C, Kennedy S, Uto H, Yamauchi K, Kobayashi M, Yusa S, Muller U, Hayashi N, Miyazaki T . Association of AIM, a novel apoptosis inhibitory factor, with hepatitis via supporting macrophage survival and enhancing phagocytotic function of macrophages . J Biol Chem 2001 ; 276 : 22910 - 22914
Kang TH, Bae K-H, Yu M-j, Kim WK, Hwang H-R, Jung H, Lee PY, Kang S, Yoon T-S, Park SG, Ryu SE, Lee SC . Phosphoproteomic analysis of neuronal cell death by glutamate-induced oxidative stress . Proteomics 2007 ; 7 : 2624 - 2635
Kang S, Kim EY, Bahn YJ, Chung JW, Lee DH, Park SG, Yoon T-S, Park BC, Bae K-H . A proteomic analysis of the effect of MAPK pathway activation on L-glutamate-induced neuronal cell death . Cell Mol Biol Lett 2007 ; 12 : 139 - 147
Kim YJ, Varki A . Perspectives on the significance of altered glycosylation of glycoproteins in cancer . Glycoconj J 1997 ; 14 : 569 - 576
Kim Y-S, Hwang SY, Oh S, Sohn H, Kang HY, Lee JH, Cho EW, Kim JY, Yoo JS, Kim CH, Miyoshi E, Taniguchi N, Ko JH . Identification of target proteins of N-acetylglucosaminyl-transferase V and fucosyltransferase 8 in human gastric tissues by glycomic approach . Proteomics 2004 ; 4 : 3353 - 3358
Kuwata K, Watanabe H, Jiang SY, Yamamoto T, Tomiyama-Miyaji C, Abo T, Miyazaki T, Naito M . AIM inhibits apoptosis of T cells and NKT cells in Corynebacterium-induced granuloma formation in mice . Am J Pathol 2003 ; 162 : 837 - 847
Lee EJ, Kim SH, Kwak YE, Kim J . Heterogeneous nuclear ribonuclear protein C is increased in the celecoxib-induced growth inhibition of human oral squamous cell carcinoma . Exp Mol Med 2006 ; 38 : 203 - 209
Lee Y, Park H-W, Park SG, Cho S, Park BC, Lee DH . Proteomic analysis of glutamate-induced toxicity in HT22 cells . Proteomics 2007 ; 7 : 185 - 193
Lovestone S, Guntert A, Hye A, Lynham S, Thambisetty M, Ward M . Proteomics of Alzheimer's disease: understanding mechanisms and seeking biomarkers . Expert Rev Proteomics 2007 ; 4 : 227 - 238
Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, Hijnen DJ, Plotz S, Knol EF, Kapp A, Bruijnzeel-Koomen CA, Ring J, de Bruin-Weller MS . Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis . Allergy 2005 ; 60 : 693 - 696
Ripolles Piquer B, Nazih H, Bourreille A, Segain JP, Huvelin JM, Galmiche JP, Bard JM . Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: consequences on the cholesterol efflux capacity of serum using Fu5AH cell system . Metabolism 2006 ; 55 : 980 - 988
Ryu SI, Kim WK, Cho HJ, Lee PY, Jung H, Yoon TS, Moon JH, Kang S, Poo H, Bae KH, Lee SC . Phosphoproteomic analysis of AML14.3D10 cell line as a model system of eosinophilia . J Biochem Mol Biol 2007 ; 40 : 765 - 772
Sarrias MR, Padilla O, Monreal Y, Carrascal M, Abian J, Vivers J, Yelamos J, Lozano F . Biochemical characterization of recombinant and circulating human Spα . Tissue Antigens 2004 ; 63 : 335 - 344
Sarrias MR, Rosello S, Sanchez-Barbero F, Sierra JM, Vila J, Yelamos J, Casals C, Lozano F . A role for human Spα as a pattern recognition receptor . J Biol Chem 2005 ; 280 : 35391 - 35398
Scirica CV, Gold DR, Ryan L, Abulkerim H, Celedon JC, Platts-Mills TA, Naccara LM, Weiss ST, Litonjua AA . Predictors of cord blood IgE levels in children at risk for asthma and atopy . J Allergy Clin Immunol 2007 ; 119 : 81 - 88
Simon D, Braathen LR, Simon H-U . Eosinophils and atopic dermatitis . Allergy 2004 ; 59 : 561 - 570
Simon D, Simon H-U . Eosinophilic disorders . J Allergy Clin Immunol 2007 ; 119 : 1291 - 1300
Simon HU . Regulation of apoptosis in diseases associated with eosinophilia . Resp Med 2000 ; 94 : 1258 - 1259
Trautmann A, Akdis M, Blaser K, Akdis CA . Role of dysregulated apoptosis in atopic dermatitis . Apoptosis 2000 ; 5 : 425 - 429
Trautmann A, Akdis M, Klunker S, Blaser K, Akdis CA . Role of apoptosis in atopic dermatitis . Int Arch Allergy Immunol 2001 ; 124 : 230 - 232
Wu J, Kobayashi M, Sousa EA, Liu W, Cai J, Goldman SJ, Dorner AJ, Projan SJ, Kavuru MS, Qiu Y, Thomassen MJ . Mol Cell Proteomics 2005 ; 4 : 1251 - 1264
Yoon SW, Kim TY, Sung MH, Kim CJ, Poo H . Comparative proteomic analysis of peripheral blood eosinophils from healthy donors and atopic dermatitis patients with eosinophilia . Proteomics 2005 ; 5 : 1987 - 1995
Yuk JS, Ha KS . Proteomic applications of surface plasmon resonance biosensors: analysis of protein arrays . Exp Mol Med 2005 ; 37 : 1 - 10
Yusa S, Ohnishi S, Onodera T, Miyazaki T . AIM, a murine apoptosis inhibitory factor, induces strong and sustained growth inhibition of B lymphocytes in combination with TGF-β1 . Eur J Immunol 1998 ; 29 : 1086 - 1093
Zhao J, Simeone DM, Heidt D, Anderson MA, Lubman DM . Comparative serum glycoproteomics using lectin selected sialic acid glycoproteins with mass spectrometric analysis: application to pancreatic cancer serum . J Proteome Res 2006 ; 5 : 1792 - 1802
Acknowledgements
We thank Drs. Haryoung Poo and Jin Woong Chung for carefully reading this manuscript. This work was supported by a grant from KRIBB (to K.-H. Bae), Korea Research Council of Fundamental Science and Technology (to K.-H. Bae), and a Molecular and Cellular BioDiscovery Research Program (to S.C. Lee) of the Korean government (MOST), Korea.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kim, W., Hwang, HR., Kim, D. et al. Glycoproteomic analysis of plasma from patients with atopic dermatitis: CD5L and ApoE as potential biomarkers. Exp Mol Med 40, 677–685 (2008). https://doi.org/10.3858/emm.2008.40.6.677
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3858/emm.2008.40.6.677
Keywords
This article is cited by
-
Apoptosis inhibitor of macrophage (AIM)/CD5L is involved in the pathogenesis of COPD
Respiratory Research (2023)
-
The macrophage soluble receptor AIM/Api6/CD5L displays a broad pathogen recognition spectrum and is involved in early response to microbial aggression
Cellular & Molecular Immunology (2014)
-
Impacts of the apoptosis inhibitor of macrophage (AIM) on obesity-associated inflammatory diseases
Seminars in Immunopathology (2014)
-
N-Glycosidase treatment with 18O labeling and de novo sequencing argues for flagellin FliC glycopolymorphism in Pseudomonas aeruginosa
Analytical and Bioanalytical Chemistry (2013)