Abstract
Mutations in the cyclin-dependent kinase-like 5 (CDKL5) gene have been described in girls with Rett-like features and early-onset epileptic encephalopathy including infantile spasms. Milder phenotypes have been associated with sequence variations in the 3′-end of the CDKL5 gene. Identification of novel CDKL5 transcripts coding isoforms characterized by an altered C-terminal region strongly questions the eventual pathogenicity of sequence variations located in the 3′-end of the gene. We investigated a group of 30 female patients with a clinically heterogeneous phenotype ranging from nonspecific intellectual disability to a severe neonatal encephalopathy and identified two heterozygous CDKL5 missense mutations, the previously reported p.Val999Met and the novel mutation p.Pro944Thr. However, these mutations have also been detected in their healthy father. Considering our results and all data from the literature, we suggest that genetic variations beyond the codon 938 in human CDKL5115 protein may have minor or no significance. It is probable that screening of exons 19–21 of the CDKL5 gene is not useful in practical molecular diagnosis of atypical Rett syndrome.
Similar content being viewed by others
Introduction
Mutations in the cyclin-dependent kinase-like 5 (CDKL5) gene (OMIM#300203) have been described in girls with Rett-like features and early-onset epileptic encephalopathy including infantile spasms.1 To date, with >80 reported cases, the phenotype of CDKL5-related encephalopathy is better defined. The main features consist of early-onset seizures starting before 5 months of age, severe mental retardation with absent speech, and Rett-like features such as hand stereotypies and deceleration of head growth. Nevertheless, milder phenotypes have been associated with sequence variations in the C-terminus of the CDKL5 gene, described by the authors as potential mutations.2, 3, 4 These potential mutations were identified in patients carrying mutations inherited from either the mother or the father, and/or in patients with mental retardation of different degrees without regression or further symptoms.4 On the other hand, three independent groups have recently identified novel CDKL5 transcripts coding isoforms characterized by an altered C-terminal region.5, 6, 7 This finding strongly questions the eventual pathogenicity of sequence variations located in the C-terminus of the gene.
Patients and methods
We investigated a group of 30 female patients with a clinically heterogeneous phenotype ranging from nonspecific mental retardation to a severe neonatal encephalopathy but no Hanefeld variant.8 Individual written consent was obtained from all sampled individuals or their legal guardians. The complete coding region of the CDKL5 gene (exons 2–21; GenBank accession number NM_003159) was screened for mutations using direct sequencing and multiplex ligation-dependent probe amplification.
Results
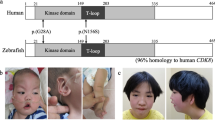
We only found two heterozygous CDKL5 missense mutations, the previously reported p.Val999Met and the novel mutation p.Pro944Thr (Figure 1).9 The p.Val999Met mutation (c.2995G>A) was found in an 11-year-old girl with moderate developmental delay/intellectual disability, speech delay, stereotypies, strabismus, mildly dysmorphic appearance, and aggressive behaviour, but her asymptomatic father also had this variant. Since DNA analysis of CDKL5, a 466-kb deletion involving the SOX5 gene was identified by CGH-array in this affected girl. In a comparable manner, the p.Pro944Thr mutation (c.2830C>A) was found in a 2-year-old girl with a severe encephalopathy. This child presented with severe developmental delay and seizures with apneas that started at the age of 3 months without spikes on electroencephalogram. At the age of 9 months, there were many tonic nocturnal seizures. From 14 months she presented with clusters of spasms and atypical hypsarrhythmia. Moreover, she exhibited slight dysmorphia and manual stereotypies. However, this sequence variation located in exon 20 has also been detected in her healthy father. This p.Pro944Thr amino-acid change affects the C-terminal part of the long CDKL5115 protein (GenBank accession number CAI42485) but not the CDKL5107 isoform (with an alternative C-terminus that terminates in intron 18).
Discussion
The human CDKL5 gene occupies ∼240 kb of the Xp22 region and is composed of 24 exons of which the first three (exons 1, 1a, and 1b) are untranslated. Two splicing variants with distinct 5′UTRs have been found: isoform I, containing exon 1, is transcribed in a wide range of tissues, whereas the expression of isoform II, including exons 1a and 1b, is limited to testis and foetal brain.5, 10 Alternative splicing events lead to at least three distinct human protein isoforms. The original CDKL5 transcript generates a long protein of 1030 amino acids (CDKL5115; 115 kDa). Although CDKL5115 is expressed mainly in testis, two other recently identified transcripts are likely to be relevant for CDKL5 brain functions.5, 7 The first one was described by Fichou et al7 after the identification of an additional 123-bp exon between exons 16 and 17 of the CDKL5 gene, referred to as CDKL5 exon 16b or CDKL5 exon 16a.6 The second one was described by Williamson et al5 after aligning both the human and mouse CDKL5 proteins to the orthologs of other species. This new isoform (GenBank accession number HQ171445) has an alternative C-terminus that terminates in intron 18, suggesting that the first nucleotides of intron 18 can be translated to produce the CDKL5107 isoform. Only CDKL5115 contains the primate-specific exons 19–21. In a similar way, a simulation software (that is, ECgene analysis; http://www.genome.ewha.ac.kr/ECgene) found another transcript with alternative splicing at exons 18 and 20 resulting in the skipping of exon 19, thus predicting a protein containing 971 amino acids. These findings raise the pathogenicity of sequence variations previously identified in the C-terminal region of CDKL5115 and the need to screen exons 19–21 in molecular diagnosis of atypical Rett syndrome.
Combining this report and the literature, two nonsense mutations (p.Arg952X and p.Arg970X), five missense mutations (p.Val966Ile, p.Val999Met, p.Ala1011Val, p.Val1013Ile, and p.Pro944Thr; Table 1), and one deletion involving the C-terminus of CDKL5 (exons 20–21) have been described beyond the point from which the two isoforms CDKL5115 and CDKL5107 diverge (Figure 2). As truncations of the C-terminus seem to enhance autophosphorylation and kinase activity of CDKL5 and cause its nuclear accumulation and activity, it has been suggested that these sequence variations may be pathogenic.11 On the basis of this line of evidence, a nonsense mutation p.Arg970X (c.2908C>T) was reported in 2009 in a girl, suggestive of ‘atypical’ Rett syndrome (severe intellectual disability, microcephaly, poor eye fixation, stereotypies, not able to walk, breathing irregularities, mild seizures at 17 months of age) but without regression, and was suggested to be pathogenic.2 Although the p.Arg970X was not found in the mother of the proband, DNA analysis in her father and a further functional study were not performed. Two years later, another nonsense mutation p.Arg952X (c.2854C>T) located in exon 20 was identified in a 15-year-old girl with generalized tonic clonic seizure provoked by fever, microcephaly, and mild intermittent exotropia of left eye without other Rett-like symptoms.3 However, identification of this variation in her healthy half sister and grandmother strongly suggested that the p.Arg952X is a rare genetic variant.3 Moreover, to date, five missense mutations have been identified in the 3′-end of CDKL5 (p.Val966Ile, p.Val999Met, p.Ala1011Val, p.Val1013Ile, and p.Pro944Thr; this study; Table 1).4, 9, 12 Three of these missense mutations have been described in Indian cases of Rett syndrome: p.Val999Met, p.Val966Ile, and p.Ala1011Val. Although these mutations appeared to be de novo, none were conserved across species and all had a low pathogenicity PolyPhen-2 score (see Table 1).12 In addition, the p.Val999Met mutation was previously described in an asymptomatic woman without skewed X-chromosome inactivation.9 During the last 5 years, we have identified this sequence variation in 17 affected individuals including 1 male patient with syndromic intellectual disability. In 12 cases, parental DNA was not analysed and the de novo nature of this sequence variation was therefore not proven. This sequence variation was inherited from the healthy mother in three cases, and from the healthy father in two independent cases (this study and unpublished data), strongly suggesting that this sequence variation is not pathogenic. The p.Val1013Ile mutation (c.3037G>A) was identified in a patient with pervasive developmental disorder during the screening of a large cohort of patients with autism spectrum disorder.4 Once again, the parental DNA analysis suggested that this sequence variation had not occurred de novo. Finally, in this report, by screening a girl with severe developmental delay and seizures, we detected the novel p.Pro944Thr amino-acid change, c.2830C>A located in exon 20. Analysis of parental DNA was performed and showed the healthy father to be a carrier of the same nucleotide change. We therefore consider this amino-acid change to be also a rare genetic variant.
Schematic representation of CDKL5. (a) Schematic representation of the human CDKL5 gene. CDKL5 exons are indicated by boxes. The three non-coding exons are shown hatched, and the new exon 16b is shown in dark grey. Mutations/variants in the 3′-end of CDKL5 reported to date are indicated corresponding to their location within the gene. (b) Human CDKL5 protein isoforms differing in the C-terminal region. Functional domains and signatures are indicated: ATP-binding domain (aa 19–43); serine threonine kinase active site (aa 131–143); TEY motif (aa 169–171), putative signal peptidase I serine active site (aa 971–978). CDKL5115 contains the primate-specific exons 19–21. In CDKL5107, 170 nucleotides of intron 18 are retained.
Considering all these data, for all these sequence variations located in exons 19–21 of the CDKL5 gene, we cannot assume that they are pathogenic. To reinforce this notion, in 2000, Huopaniemi et al13 described a large 136-kb inherited deletion, truncating the 3′-end of the CDKL5 gene (including the 3′-end of exon 20 and 21) and including both the RS1 and PPEF1 genes in a male with retinoschisis, mild hemiplegia, and epilepsy, but not the Hanefeld variant.
In conclusion, all the genetic data obtained in patients who carry a mutation at a position farther than 938th amino acid suggest that these sequence variations located in exons 19–21 are rare polymorphisms. The most 3′-end mutations affecting CDKL5107 isoform are located in exon 18 (c.2635_2636delCT (p.Glu879fsX908) and p.Gln834X) and are identified in patients with atypical Rett syndrome. The recurrent c.2635_2636delCT mutation was found in an 8-year-old girl with severe intellectual disability, poor eye fixation, hand mouthing, no walk, no speech, and seizures, and in a 2-year-old girl with regression, severe intellectual disability, poor eye fixation, stereotypies, hand apraxia, and seizures.8, 14 The p.Gln834X mutation was found in a 4-year-old girl with severe intellectual disability, stereotypies, hand apraxia, and seizures.9
As the theoretical 107-kDa isoform appeared to be the major stable isoform in human brain,5 and considering that the mouse cdkl5 protein contains only 938 amino acids, we can assume that genetic variations beyond the codon 938 in human CDKL5115 protein may have minor or no significance. It is likely that screening of exons 19–21 of the CDKL5 gene is not useful in practical molecular diagnosis of atypical Rett syndrome.
References
Bahi-Buisson N, Bienvenu T : CDKL5-related disorders: from clinical description to molecular genetics. Mol Syndromol 2012; 2: 137–152.
Psoni S, Willems PJ, Kanavakis E et al: A novel p.Arg970X mutation in the last exon of the CDKL5 gene resulting in late-onset seizure disorder. Eur J Paediatr Neurol 2010; 14: 188–191.
Intusoma U, Hayeeduereh F, Plong-On O et al: Mutation screening of the CDKL5 gene in cryptogenic infantile intractable epilepsy and review of clinical sensitivity. Eur J Paediatr Neurol 2011; 15: 432–438.
Schaaf CP, Sabo A, Sakai Y et al: Oligogenic heterozygosity in individuals with high- functioning autism spectrum disorders. Hum Mol Genet 2011; 20: 3366–3375.
Williamson SL, Giudici L, Kilstrup-Nielsen C et al: A novel transcript of cyclin-dependent kinase-like 5 (CDKL5) has an alternative C-terminus and is the predominant transcript in brain. Hum Genet 2012; 131: 187–200.
Rademacher N, Hambrock M, Fischer U et al: Identification of a novel CDKL5 exon and pathogenic mutations in patients with severe mental retardation, early-onset seizures and Rett-like features. Neurogenetics 2011; 12: 165–167.
Fichou Y, Nectoux J, Bahi-Buisson N, Chelly J, Bienvenu T : An isoform of the severe encephalopathy-related CDKL5 gene, including a novel exon with extremely high sequence conservation, is specifically expressed in brain. J Hum Genet 2011; 56: 52–57.
Bahi-Buisson N, Nectoux J, Rosas-Vargas H et al: Key clinical features to identify girls with CDKL5 mutations. Brain 2008; 131: 2647–2661.
Nectoux J, Heron D, Tallot M, Chelly J, Bienvenu T : Maternal origin of a novel C-terminal truncation mutation in CDKL5 causing a severe atypical form of Rett syndrome. Clin Genet 2006; 70: 29–33.
Kalscheuer VM, Tao J, Donnelly A et al: Disruption of the serine/threonine kinase 9 gene causes severe X-linked infantile spasms and mental retardation. Am J Hum Genet 2003; 72: 1401–1411.
Rusconi L, Salvatoni L, Giudici L et al: CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail. J Biol Chem 2008; 283: 30101–30111.
Das DK, Mehta B, Menon SR, Raha S, Udani V : Novel mutations in cyclin-dependent kinase-like 5 (CDKL5) gene in Indian cases of Rett syndrome. Neuromol Med 2012; 15: 218–225.
Huopaniemi L, Tyynismaa H, Rantala A, Rosenberg T, Alitalo T : Characterization of two unusual RS1 gene deletions segregating in Danish retinoschisis families. Hum Mutat 2000; 16: 307–314.
Scala E, Ariani F, Mari F et al: CDKL5/STK9 is mutated in Rett syndrome variant with infantile spasms. J Med Genet 2005; 42: 103–107.
Acknowledgements
This work was supported by grant from Fondation Jérome Lejeune.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Diebold, B., Delépine, C., Gataullina, S. et al. Mutations in the C-terminus of CDKL5: proceed with caution. Eur J Hum Genet 22, 270–272 (2014). https://doi.org/10.1038/ejhg.2013.133
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2013.133
Keywords
This article is cited by
-
Molecular and genetic insights into an infantile epileptic encephalopathy – CDKL5 disorder
Frontiers in Biology (2017)