Abstract
Background:
Resting metabolic rate (RMR) should be measured in the thermoneutral zone (TNZ). Forearm to fingertip skin temperature gradients (FFG) could serve as an objective measure of this pre-condition.
Subjects/Methods:
Eighty-six adult Australians were studied at 25 °C in a temperature-controlled chamber. Measurements of overnight fasted RMR, respiratory quotient (RQ) and FFG were complemented by clinical biochemistry. McAuley’s Index of insulin sensitivity (McA_ISI) and presence of metabolic syndrome was determined. Physical activity was estimated from the short version of the International Physical Activity Questionnaire. Fat mass (FM) and fat-free mass (FFM) were obtained from dual-energy x-ray absorptiometry. Twenty-nine participants were assessed for changes in RMR (ΔRMR), RQ (ΔRQ) and FFG (ΔFFG) following a 6-month free-living period. Multiple linear regression analyses of RMR and RQ on FFG, and of ΔRMR and ΔRQ on ΔFFG were conducted after controlling for 12 known determinants of energy metabolism.
Results:
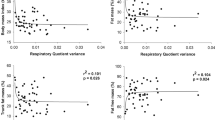
There were wide between-subject variations in unadjusted FFG ranging from −4.25 to +7.8 °C. The final parsimonious model for cross-sectional observations of RMR included age, FM, FFM, McA_ISI and FFG (β=63 kJ/d (95% confidence interval (CI): 14.2, 112.1, P=0.012)). However, FFG was unrelated to RQ.
In the longitudinal cohort, adjusted ΔRMR significantly associated only with ΔFFG (β=100 kJ/d (95% CI: 10.3, 189.1; P=0.030)), and adjusted ΔRQ associated with ΔFFG (−0.003 (95% CI: −0.005, 0.0002, P=0.038)), age and McA_ISI.
Conclusions:
Sizeable between-subject variations in FFG at 25 °C were associated with RMR and RQ. Monitoring FFG may serve as an objective assessment of the TNZ during RMR measurements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Clapham J . Central control of thermogenesis. Neuropharmacology 2012; 63: 1–13.
Soares M, Piers LS, Kraai L, Shetty PS . Day-to-day variations in basal metabolic rates and energy intakes of human subjects. Eur J Clin Nutr 1989; 43: 465–472.
Johnstone A, Murison SD, Duncan JS, Rance KA, Speakman JR . Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am J Clin Nutr 2005; 82: 941–948.
Cunningham J . A reanalysis of the factors influencing basal metabolic rate in normal adults. Am J Clin Nutr 1980; 33: 2372–2374.
Van Pelt RE, Dinneno FA, Seals DR . Age-related decline in RMR in physically active men: relation to exercise volume and energy intake. Am J Physiol Endocrinol Metab 2001; 281: E633–E639.
Landsberg L, Young JB, Leonard WR, Linsenmeier RA, Turek FW . Do the obese have lower body temperatures? A new look at a forgotten variable in energy balance. Trans Am Clin Climatol Assoc 2009; 120: 287–295.
Muller MJ, Bosy-Westphal A, Later W, Haas V, Heller M . Functional body composition: insights into the regulation of energy metabolism and some clinical applications. Eur J Clin Nutr 2009; 63: 1045–1056.
Nsatimba P, Pathak K, Soares MJ . Ethnic differences in resting metabolic rate, respiratory quotient and body temperature: a comparison of Africans and European Australians. Eur J Nutr 2016; 55: 1831–1838.
Hayter JR, Henry CJ . Basal metabolic rate in human subjects migrating between tropical and temperate regions: a longitudinal study and review of previous work. Eur J Clin Nutr 1993; 47: 724–734.
Gallagher D, Albu J, He Q, Heshka S, Boxt L, Krasnow N et al. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr 2006; 83: 1062–1067.
Kimm SY, Glynn NW, Aston CE, Damcott CM, Poehlman ET, Daniels SR et al. Racial differences in the relation between uncoupling protein genes and resting energy expenditure. Am J Clin Nutr 2002; 75: 714–719.
Calton EK, Pathak K, Soares MJ, Alfonso H, Keane KN, Newsholme P et al. Vitamin D status and insulin sensitivity are novel predictors of resting metabolic rate: a cross-sectional analysis in Australian adults. Eur J Nutr 2016; 55: 2075–2080. doi: 10.1007/s00394-015-1021-z.
Soares M, Cummings NK, Chan She Ping-Delfos WL . Energy metabolism and the metabolic syndrome: does a lower basal metabolic rate signal recovery following weight loss? Diabetes Metab Syndr 2011; 5: 98–101.
Blaak E . Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 2001; 4: 499–502.
Landsberg L, Saville ME, Young JB . Sympathoadrenal system and regulation of thermogenesis. Am J Physiol Endocrinol Metab 1984; 247: E181–E189.
Van-Den-Berg S, Berga VD, van Marken Lichtenbelt W, van Dijka KW, Schrauwen P . Skeletal muscle mitochondrial uncoupling, adaptive thermogenesis and energy expenditure. Curr Opin Clin Nutr Metab Care 2011; 14: 243–249.
Claessens–van Ooijen AMJ Human Thermoregulation: Individual Differences in Cold Induced Thermogenesis (PhD). Universiteit Maastricht: Eindhoven, The Netherlands, 2008..
Calton E, Soares MJ, James AP, Woodman RJ . The potential role of irisin in the thermoregulatory responses to mild cold exposure in adults. Am J Hum Biol 2016; 28: 699–704.
Roth G, Sheard C . Relation of basal metabolic rate to vasodilatation and vasoconstriction of the extremities of normal subjects as measured by skin temperatures. Circulation 1950; 1: 1142–1147.
Van Ooijena A, van Marken Lichtenbelta WD, van Steenhovenb AA, Westerterpa KR . Seasonal changes in metabolic and temperature responses to cold air in humans. Physiol Behav 2004; 82: 545–553.
Kingma B, Frijns AJH, Schellen L, van Marken Lichtenbelt WD . Beyond the classic thermoneutral zone: including thermal comfort. Temperature 2014; 1: 142–149.
Kingma B, Frijns A, van Marken Lichtenbelt W . The thermoneutral zone: implications for metabolic studies. Front Biosci 2012; E4: 1975–1985.
Henry C . Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 2006; 8: 1133–1152.
Faul F, Erdfelder E, Lang AG, Buchner A . G*Power 3: a flexible statistical power 290 analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191.
McAuley K, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA et al. Diagnosing insulin resistance in the general population. Diabetes Care 2001; 24: 460–464.
Alberti KGMM, Zimmet P, Shaw J . Metabolic syndrome- a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006; 23: 469–480.
Lee P, Macfarlane DJ, Lam TH, Stewart SM . Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 2011; 8: 115.
O’Brien RM . A caution regarding rules of thumb for variance inflation factors. Qual Quant 2007; 41: 673–690.
Calton EK, Pathak K, Soares MJ, Alfonso H, Keane KN, Newsholme P et al. Vitamin D status and insulin sensitivity are novel predictors of resting metabolic rate: a cross-sectional analysis in Australian adults. Eur J Nutr 2016; 55: 2075–2080.
Ravussin E, Bogardus C . Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr 1989; 49 (5 Suppl), 968–975.
Du Bois EF . The basal metabolism in fever. J Am Med Assoc 1921; 77: 352–355.
Caldwell JN, Matsuda-Nakamura M, Taylor NA . Three-dimensional interactions of mean body and local skin temperatures in the control of hand and foot blood flows. Eur J Appl Physiol 2014; 114: 679–689.
Rubinstein E, Sessler DI . Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology 1990; 73: 541–545.
House JR, Tipton MJ . Using skin temperature gradients or skin heat flux measurements to determine thresholds of vasoconstriction and vasodilatation. Eur J Appl Physiol 2002; 88: 141–145.
Muller M, Bosy-Westphal A, Later W, Haas V, Heller M . Functional body composition: insights into the regulation of energy metabolism and some clinical applications. Eur J Clin Nutr 2009; 60: 1045–1056.
Wijers SLJ, Saris WHM, van Marken Lichtenbelt WD . Cold-induced adaptive thermogenesis in lean and obese. Obesity 2010; 18: 1092–1099.
Maeda T, Fukushima T, Ishibashi K, Higuchi S . Involvement of basal metabolic rate in determination of type of cold tolerance. J Physiol Anthropol 2007; 26: 415–418.
Acknowledgements
Author contributions
MJS generated the idea. KP and EKC conducted the studies. MJS verified the data entry and collation. YZ planned the statistical analysis, cross-checked statistical outputs and critically reviewed the manuscript. KP analyzed the data and wrote the first draft with MJS. TJ, KK and PN critically reviewed the manuscript. All authors read and contributed to the final written manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pathak, K., Calton, E., Soares, M. et al. Forearm to fingertip skin temperature gradients in the thermoneutral zone were significantly related to resting metabolic rate: potential implications for nutrition research. Eur J Clin Nutr 71, 1074–1079 (2017). https://doi.org/10.1038/ejcn.2017.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2017.30
This article is cited by
-
Resting energy expenditure and body composition: critical aspects for clinical nutrition
European Journal of Clinical Nutrition (2018)