Abstract
Background/Objectives:
Exclusive enteral nutrition (EEN) is a safe and effective treatment modality for inducing remission in paediatric Crohn’s disease (CD). The primary aim of this study was to compare the outcomes of EEN to corticosteroid (CS) therapy in newly diagnosed, treatment-naïve patients with CD. A secondary aim was to describe the outcomes of EEN in a national cohort of paediatric CD patients over a 10-year period.
Subjects/Methods:
A retrospective chart review was conducted at the Irish national referral centre for paediatric CD. A case-matched analysis was conducted on two cohorts matched for age, gender, disease location, disease behaviour and disease activity, who received CS or EEN as their initial treatment. Subsequently, cohort analysis was conducted on all patients who undertook a course of EEN therapy between 2004 and 2013.
Results:
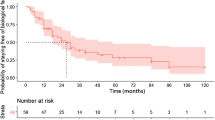
The case-matched analysis found higher remission rates after treatment with EEN (24/28, 86%) compared with those with CS (15/28, 54%; P=0.02). Dietetic contacts were found to be pivotal to the success of treatment and the attainment of remission. In total, 59 patients completed EEN at some time-point in their disease course and were included in the cohort analysis. Sixty-nine per cent of this cohort entered clinical remission (41/59). EEN was found to be most effective when used as an initial treatment (P=0.004) and less effective in patients aged under 10 years (P=0.04).
Conclusions:
EEN should be strongly considered as a favourable primary treatment over CS, especially in those diagnosed over the age of 10 years.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sandhu BK, Fell JME, Beattie RM, Mitton SG, Wilson DC, Jenkins H et al. Guidelines for the management of inflammatory bowel disease in children in the United Kingdom. J Pediatr Gastroenterol Nutr 2010; 50: S1–13.
Frivolt K, Schwerd T, Werkstetter KJ, Schwarzer A, Schatz SB, Bufler P et al. Repeated exclusive enteral nutrition in the treatment of paediatric Crohn's disease: predictors of efficacy and outcome. Aliment Pharmacol Ther 2014; 39: 1398–1407.
Gerasimidis K, McGrogan P, Edwards CA . The aetiology and impact of malnutrition in paediatric inflammatory bowel disease. J Hum Nutr Diet 2011; 24: 313–326.
Moeeni V, Day AS . Nutritional risk screening tools in hospitalised children. Int J Child Health Nutr 2012; 1: 39–43.
Ezri J, Marques-Vidal P, Nydegger A . Impact of disease and treatments on growth and puberty of pediatric patients with inflammatory bowel disease. Digestion 2012; 85: 308–319.
Day A, Ledder O, Leach S, Lemberg D . Crohn's and colitis in children and adolescents. World J Gastroenterol 2012; 18: 5862–5869.
Hope B, Shahdadpuri R, Dunne C, Broderick AM, Grant T, Hamzawi M et al. Rapid rise in incidence of Irish paediatric inflammatory bowel disease. Arch Dis Child 2012; 97: 590–594.
Wall CL, Day AS, Gearry RB . Use of exclusive enteral nutrition in adults with Crohn’s disease: a review. World J Gastroenterol 2013; 19: 7652–7660.
Day A, Burgess L . Exclusive enteral nutrition and induction of remission of active Crohn’s disease in children. Expert Rev Clin Immunol 2013; 9: 375–383.
Cameron FL, Gerasimidis K, Papangelou A, Missiou D, Garrick V, Cardigan T et al. Clinical progress in the two years following a course of exclusive enteral nutrition in 109 paediatric patients with Crohn’s disease. Aliment Pharmacol Ther 2013; 37: 622–629.
Pigneur B, Seksik P, Viola S, Viala J, Beaugerie L, Girardet JP et al. Natural history of Crohn’s disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010; 16: 953–961.
De Bie C, Kindermann A, Escher J . Use of exclusive enteral nutrition in pediatric Crohn’s disease in the Netherlands. J Crohn's Colitis 2013; 7: 263–270.
Day A, Whitten K, Sidler M, Lemberg D . Systematic review: nutritional therapy in paediatric Crohn’s disease. Aliment Pharmacol Ther 2008; 27: 293–307.
Whitten KE, Rogers P, Ooi CKY, Day AS . International survey of enteral nutrition protocols used in children with Crohn’s disease. J Dig Dis 2012; 13: 107–112.
Kansal S, Wagner J, Kirkwood CD, Catto-Smith AG . Enteral nutrition in Crohn’s disease: an underused therapy. Gastroenterol Res Pract 2013; 2013: 482108.
Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis 2014; 8: 1179–1207.
Heuschkel R, Menache C, Megerian J, Baird A . Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr 2000; 31: 8–15.
Soo J, Malik BA, Turner JM, Persad R, Wine E, Siminoski K et al. Use of exclusive enteral nutrition is just as effective as corticosteroids in newly diagnosed pediatric Crohn’s disease. Dig Dis Sci 2013; 58: 3584–3591.
Dziechciarz P, Horvath A, Shamir R, Szajewska H . Meta-analysis: enteral nutrition in active Crohn’s disease in children. Aliment Pharmacol Ther 2007; 26: 795–806.
Zachos M, Tondeur M, Griffiths AM . Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2007; 1: CD000542.
Afzal NA, Van Der Zaag-Loonen HJ, Arnaud-Battandier F, Davies S, Murch S, Derkx B et al. Improvement in quality of life of children with acute Crohn’s disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment Pharmacol Ther 2004; 20: 167–172.
Berni Canani R, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A et al. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis 2006; 38: 381–387.
Borrelli O, Cordischi L, Cirulli M, Paganelli M, Labalestra V, Uccini S et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol 2006; 4: 744–753.
Werkstetter KJ, Schatz SB, Alberer M, Filipiak-Pittroff B, Koletzko S . Influence of exclusive enteral nutrition therapy on bone density and geometry in newly diagnosed pediatric Crohn's disease patients. Ann Nutr Metab 2013; 63: 10–16.
Grover Z, Muir R, Lewindon P . Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J Gastroenterol 2014; 49: 638–645.
Sidoroff M, Kolho KL . Glucocorticoids in pediatric inflammatory bowel disease. Scand J Gastroenterol 2012; 47: 745–750.
IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). Medical Position Paper: Inflammatory Bowel Disease in Children and Adolescents: Recommendations for Diagnosis—The Porto Criteria. J Pediatr Gastroenterol Nutr 2005; 41: 1–7.
Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011; 17: 1314–1321.
Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS et al. Development and validation of a Pediatric Crohn's Disease Activity Index. J Pediatr Gastroenterol Nutr 1991; 12: 439–447.
Hyams J, Markowitz J, Otley A, Rosh J, Mack D, Bousvaros A et al. Evaluation of the Pediatric Crohn Disease Activity Index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr 2005; 41: 416–421.
Great Ormond Street Dietetic Department. Great Ormond Street Dietetic Department. Nutritional Requirements for Children in Health and Disease, 3rd edn. Great Ormond Street Hospital for Children, NHS Trust: London, UK, 2005.
Ireland Central Statistics Office. Small area population statistics interactive mapping tool. Census 2011. Available at http://www.cso.ie/en/census/census2011smallareapopulationstatisticssaps/.
Buchanan E, Gaunt WW, Cardigan T, Garrick V, McGrogan P, Russell RK . The use of exclusive enteral nutrition for induction of remission in children with Crohn’s disease demonstrates that disease phenotype does not influence clinical remission. Aliment Pharmacol Ther 2009; 30: 501–507.
Navas-Lopez V, Blasco-Alonso J, Maseri SL, Fernández-Crehuet FG, Nieto MJS, Recio MIV et al. Exclusive enteral nutrition continues to be first line therapy for pediatric Crohn’s disease in the era of biologics. An Pediatr (Barc) 2014; 83: 47–54.
Alhagamhmad M, Day A, Lemberg D, Leach S . An update of the role of nutritional therapy in the management of Crohn’s disease. J Gastroenterol 2012; 47: 872–882.
Day AS, Whitten KE, Lemberg DA, Clarkson C, Vitug-Sales M, Jackson R et al. Exclusive enteral feeding as primary therapy for Crohn’s disease in Australian children and adolescents: a feasible and effective approach. J Gastroenterol Hepatol 2006; 21: 1609–1614.
Rubio A, Pigneur B, Garnier-Lengliné H, Talbotec C, Schmitz J, Canioni D et al. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment Pharmacol Ther 2011; 33: 1332–1339.
Gavin J, Anderson C, Bremner A, Beattie R . Energy intakes of children with Crohn’s disease treated with enteral nutrition as primary therapy. J Hum Nutr Diet 2005; 18: 337–342.
Gupta K, Noble A, Kachelries K, Albenberg L, Kelsen J, Grossman A et al. A novel enteral nutrition protocol for the treatment of pediatric Crohn’s disease. Inflamm Bowel Dis 2013; 19: 1374–1378.
Boneh-Sigall R, Pfeffer-Gik T, Segal I, Zangen T, Boaz M, Levine A . Partial enteral nutrition with a Crohn's disease exclusion diet is effective for induction of remission in children and young adults with Crohn's disease. Inflamm Bowel Dis 2014; 20: 1353–1360.
Acknowledgements
We thank the following for their support and assistance in completing this study: Mary Hamzawi, Siobhain Kiernan, Karen O’ Driscoll, Prof. Billy Bourke, Dr Annemarie Broderick, Dr Shoana Quinn, Dr Michael Mc Dermott, Prof Maureen O’Sullivan and the DOCHAS study project. LL and MT are students from Dublin Institute of Technology and Trinity College Dublin. AC is funded by the National Children’s Research Centre as part of the DOCHAS study.
Author contributions
Study concept and design: SH, AC, MH and SS. Data collection and analysis: MT, LL and AC. Manuscript drafting: all authors. Study guarantor: SH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lafferty, L., Tuohy, M., Carey, A. et al. Outcomes of exclusive enteral nutrition in paediatric Crohn’s disease. Eur J Clin Nutr 71, 185–191 (2017). https://doi.org/10.1038/ejcn.2016.210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2016.210