Abstract
Background/Objective:
The objective of this study was to determine the effects of substitution of red meat with legumes in the Therapeutic Lifestyle Change (TLC) diet on cardiometabolic risk factors in type 2 diabetes patients based on dietary education.
Subjects/Methods:
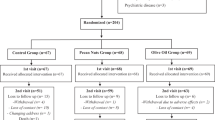
This study was a randomized, controlled, cross-over trial. Thirty-one participants (24 women and 7 men; age: 58.1±6.0 years) with type 2 diabetes were randomly assigned to consume a control diet (legume-free TLC diet) and legume-based TLC diet for 8 weeks. Legume-based TLC diet was the same as the control diet, but the legume-based TLC group was advised to replace two servings of red meat with legumes, 3 days per week. After the interventional period, a washout period was conducted for 4 weeks. The groups were then advised to follow the alternate treatment for 8 weeks. Cardiometabolic risk factors were measured.
Results:
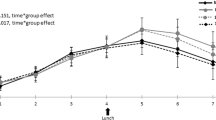
Compared with the legume-free TLC diet, the legume-based TLC diet significantly decreased fasting blood glucose (P=0.04), fasting insulin (P=0.04), triglyceride concentrations (P=0.04) and low-density lipoprotein cholesterol (P=0.02). Total cholesterol concentrations decreased after consumption of both TLC diet and legume TLC diet; however, the data did not differ significantly between the two diets. body mass index (BMI), waist circumference, systolic and diastolic blood pressures did not change significantly after consumption of either the legume-free TLC diet or the legume-based TLC diet.
Conclusions:
Dietary advice given for substitution of red meat with legume intakes within a TLC diet-improved lipid profiles and glycemic control among diabetes patients, which were independent from BMI change. This trial was registered in the Iranian Registry of Clinical Trials (http://www.irct.ir) as IRCT201202251640N7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kalofoutis C, Piperi C, Kalofoutis A, Harris F, Phoenix D, Singh J . Type II diabetes mellitus and cardiovascular risk factors: current therapeutic approaches. Exp Clin Cardiol 2007; 12: 17–28.
White DL, Collinson A . Red meat, dietary heme iron, and risk of type 2 diabetes: the involvement of advanced lipoxidation end products. Adv Nutr 2013; 4: 403–411.
Mursu J, Virtanen JK, Tuomainen TP, Nurmi T, Voutilainen S . Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2014; 99: 328–333.
Aune D, Norat T, Romundstad P, Vatten LJ . Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013; 98: 1066–1083.
Aune D, Norat T, Romundstad P, Vatten LJ . Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol 2013; 28: 845–858.
Villegas R, Gao YT, Yang G, Li HL, Elasy TA, Zheng W, Shu XO . Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr 2008; 87: 162–167.
Bazzano LA, Thompson AM, Tees MT, Nguyen CH, Winham DM . Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 2011; 21: 94–103.
Hamden K, Jaouadi B, Carreau S, Aouidet A, Elfeki A . Therapeutic effects of soy isoflavones on α-amylase activity, insulin deficiency, liver-kidney function and metabolic disorders in diabetic rats. Nat Prod Res 2011; 25: 244–255.
US Department of Agriculture, US Department of Health and Human Services Dietary Guidelines for Americans 2010: www.dietaryguidelines.gov.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the NationalCholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421.
Welty FK, Lee KS, Lew NS, Zhou JR . Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med 2007; 167: 1060–1067.
Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L . Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care 2012; 35: 1981–1985.
Institute of Medicine of the National Academies, Food and Nutrition Board. Dietary reference intakes: for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. The National Academy Press: Washington, DC 167. 2002, pp 1060–1067.
Institute of Medicine of the National Academies, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. The National Academy Press: Washington DC. 2005, pp 107–264.
Rebello CJ, Greenway FL, Finley JW . A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes Rev 2014; 15: 392–407.
Bouchenak M, Lamri-Senhadji M . Nutritional quality of legumes, and their role in cardiometabolic risk prevention: a review. J Med Food 2013; 16: 185–198.
Venter CS, Vorster HH, Cummins JH . Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am J Gastroenterol 1990; 85: 549–553.
Hokanson JE, Austin MA . Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996; 3: 213–219.
Zhang Z, Lanza E, Kris-Etherton PM, Colburn NH, Bagshaw D, Rovine MJ et al. A high legume low glycemic index diet improves serum lipid profiles in men. Lipids 2010; 45: 765–775.
Hermsdorff HH, Zulet MÁ, Abete I, Martínez JA . A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr 2011; 50: 61–69.
Crujeiras AB, Parra D, Abete I, Martínez JA . A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic Res 2007; 41: 498–506.
Ha V, Sievenpiper JL, de Souza RJ, Jayalath VH, Mirrahimi A, Agarwal A et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: a systematic review and meta-analysis of randomized controlled trials. CMAJ 2014; 186: E252–E262.
Galisteo M, Duarte J, Zarzuelo A . Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem 2008; 19: 71–84.
Rochfort S, Panozzo J . Phytochemicals for Health, the Role of Pulses. J Agric Food Chem 2007; 55: 7981–7994.
Jenkins DJ, Kendall CW, Augustin LS, Mitchell S, Sahye-Pudaruth S, Blanco Mejia S et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2012; 172: 1653–1660.
Pittaway JK, Robertson IK, Ball MJ . Chickpeas may influence fatty acid and fiber intake in an ad libitum diet, leading to small improvements in serum lipid profile and glycemic control. J Am Diet Assoc 2008; 108: 1009–1013.
Ley SH, Sun Q, Willett WC, Eliassen AH, Wu K, Pan A et al. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr 2014; 99: 352–360.
Hartman TJ, Albert PS, Zhang Z, Bagshaw D, Kris-Etherton PM, Ulbrecht J et al. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr 2010; 140: 60–67.
Nestel P, Cehun M, Chronopoulos A . Effects of long-term consumption and single meals of chickpeas on plasma glucose, insulin, and triacylglycerol concentrations. Am J Clin Nutr 2004; 79: 390–395.
Sievenpiper JL, Kendall CW, Esfahani A, Wong JM, Carleton AJ, Jiang HY et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009; 52: 1479–1495.
Martínez-González MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, Basterra-Gortari FJ, Beunza JJ, Vazquez Z et al. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ 2008; 336: 1348–1351.
Zahradka P, Wright B, Weighell W, Blewett H, Baldwin A, O K et al. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis 2013; 230: 310–314.
Abete I, Parra D, Martinez JA . Legume-, fish-, or high-protein-based hypocaloric diets: effects on weight loss and mitochondrial oxidation in obese men. J Med Food 2009; 12: 100–108.
Tovar J, Nilsson A, Johansson M, Björck I . Combining functional features of whole-grain barley and legumes for dietary reduction of cardiometabolic risk: a randomised cross-over intervention in mature women. Br J Nutr 2014; 111: 706–714.
Acknowledgements
The authors acknowledge Ms N Shiva for critical editing of English grammar and syntax of the manuscript. This study was funded by grant no. 411 from the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author Contributions
The project idea for this study was from S.H. The project was designed by P.M, S.H and F.A. S.H, and M.H analyzed and interpreted the data; S.H and P.M prepared the manuscript. All authors read and approved the final manuscript. Overall F.A supervised the project and approved the final version of the manuscript to be submitted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hosseinpour-Niazi, S., Mirmiran, P., Hedayati, M. et al. Substitution of red meat with legumes in the therapeutic lifestyle change diet based on dietary advice improves cardiometabolic risk factors in overweight type 2 diabetes patients: a cross-over randomized clinical trial. Eur J Clin Nutr 69, 592–597 (2015). https://doi.org/10.1038/ejcn.2014.228
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.228
This article is cited by
-
Plasma metabolite profile of legume consumption and future risk of type 2 diabetes and cardiovascular disease
Cardiovascular Diabetology (2024)
-
Effect of legumes in energy reduced dietary approaches to stop hypertension (DASH) diet on blood pressure among overweight and obese type 2 diabetic patients: a randomized controlled trial
Diabetology & Metabolic Syndrome (2022)
-
Improvement of glycemic indices by a hypocaloric legume-based DASH diet in adults with type 2 diabetes: a randomized controlled trial
European Journal of Nutrition (2022)
-
Therapeutic lifestyle change diet enriched in legumes reduces oxidative stress in overweight type 2 diabetic patients: a crossover randomised clinical trial
European Journal of Clinical Nutrition (2018)
-
Understanding Cultural Influences on Dietary Habits in Asian, Middle Eastern, and Latino Patients with Type 2 Diabetes: A Review of Current Literature and Future Directions
Current Diabetes Reports (2017)