Abstract
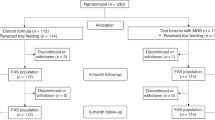
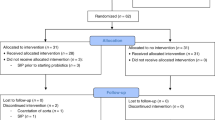
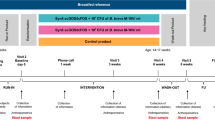
Studies undertaken with α-lactalbumin-enriched formulae never addressed infants with colic. This study evaluated the nutritional adequacy, the gastrointestinal tolerance and the effect on colic of an α-lactalbumin-enriched and probiotic-supplemented formula. A double-blind, placebo-controlled study enrolled 66 healthy infants with colic, aged 3 weeks to 3 months, fed during 1 month with the either experimental formula (EF, Modilac Digest 1) or control formula (CF) and evaluated for efficacy and safety parameters at days 15 and 30. Weight and height gains were identical in the two groups and complied with standards (1023.4±360.4 g (EF) and 1047.4±372.1 g (CF), NS; 4.2±1.4 cm (EF) and 4.3±1.9 cm (CF), NS). No differences were found between groups for crying duration. ‘Feeding-related’ gastrointestinal side effects were significantly lower with EF than with CF (P=0.011). An α-lactalbumin-enriched and probiotic-supplemented formula guaranteed good weight and length gains to infants with colic and seemed to provide good gastrointestinal tolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Cohen-Silver J, Ratnapalan S (2009). Management of infantile colic: a review. Clin Pediatr 48, 14–17.
Coppa GV, Bruni S, Morelli L, Soldi S, Gabrielli O (2004). The first prebiotics in humans: human milk oligosaccharides. J Clin Gastroenterol 38 (6 Suppl), S80–S83.
Davis AM, Harris BJ, Lien EL, Pramuk K, Trabulsi J (2008). α-Lactalbumin-rich infant formula fed to healthy term infants in a multicenter study: plasma essential amino acids and gastrointestinal tolerance. Eur J Clin Nutr 62, 1294–1301.
Heine W, Radke M, Wutzke KD, Peters E, Kundt G (1996). Alpha-lactalbumin-enriched low-protein infant formulas: a comparison to breast milk feeding. Acta Paediatr 85, 1024–1028.
Lien EL, Davis AM, Euler AR (2004). Growth and safety in term infants fed reduced-protein formula with added bovine alpha-lactalbumin. J Pediatr Gastroenterol Nutr 38, 170–176.
Savino F (2007). Focus on infantile colic. Acta Paediatr 96, 1259–1264.
Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F et al. (2006). Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. Eur J Clin Nutr 60, 1304–1310.
Savino F, Pelle E, Palumeri E, Oggero R, Miniero R (2007). Lactobacillus reuteri (American Type Culture Collection strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 119, e124–e130.
Wessel MA, Cobb JC, Jackson EB, Harris Jr GS, Detwiler AC (1954). Paroxysmal fussing in infancy, sometimes called colic. Pediatrics 14, 421–435.
World Health Organization de Onis M, Garza C, Cesar G, Maharaj K, Kaare R guest editors (2004). Multicentre Growth Reference Study (MGRS): rationale, planning, and implementation. Food Nutr Bull 25, S3–S84.
Acknowledgements
This work was financially supported by Sodilac, France. We thank the Ordesa Group for providing scientific support. The protocol was carried out by the following general paediatricians: Dr N Belaroussi Maamri, Dr F Gressin-Cohen, Dr C Grillon, Dr S Hadji, Dr N Kalach, Dr B Pacault, Dr C Rizk, Dr A Locquet and Dr K Ara.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Honoraria were granted to Professor C Dupont for coordination of the study and to all investigators, including Dr N Belaroussi, Dr F Gressin-Cohen, Dr C Grillon, Dr S Hadji, Dr N Kalach, Dr B Pacault, Dr C Rizk, Dr A Locquet and Dr K Ara.
Rights and permissions
About this article
Cite this article
Dupont, C., Rivero, M., Grillon, C. et al. α-Lactalbumin-enriched and probiotic-supplemented infant formula in infants with colic: growth and gastrointestinal tolerance. Eur J Clin Nutr 64, 765–767 (2010). https://doi.org/10.1038/ejcn.2010.81
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2010.81
Keywords
This article is cited by
-
An overview of systematic reviews of complementary and alternative therapies for infantile colic
Systematic Reviews (2019)
-
Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: a randomized, controlled trial
Pediatric Research (2015)
-
Probiotics and Prebiotics in Infants and Children
Current Infectious Disease Reports (2013)
-
Probiotics to improve outcomes of colic in the community: Protocol for the Baby Biotics randomised controlled trial
BMC Pediatrics (2012)