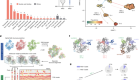
Molecular glues are small molecules that stabilize the complex formation between E3 ligases and therapeutic targets, facilitating the ubiquitination and degradation of the target proteins. These target proteins are often considered ‘undruggable’ in traditional drug design. Molecular glues prepare the surface of E3 ligase for ideal docking of the target protein, but the exact mechanism of action requires more understanding for effective molecular glue designs.
This webcast will demonstrate harnessing the power of mass photometry to analyze the oligomeric state of the DCAF15–DDA1–DDB1 E3 ligase at low nanomolar concentrations. This study revealed, for the first time, the formation of dimers and trimers with quantified dissociation constants. The oligomeric remodelling effect emphasized the transition from trimer to monomer upon molecular glue addition, shedding light on how molecular glues regulate E3 ligase oligomerization at physiological concentration. This oligomeric remodelling and ternary complex formation were also characterized and quantified by mass spectrometry. The existing weak interactions between E3 ligase and a target protein were also confirmed by mass spectrometry, which could lead to more efficient screening for E3 ligase candidates.
Learn:
• What are the applications and capabilities of mass photometry?
• How can mass photometry be used to characterize oligomerization behavior of protein complexes?
• What is the unique regulatory mechanism of molecular glues for targeted protein degradation?
Unable to join the live event? Watch on demand. Register now to ensure that you receive information on how to gain access after the live event.
This webcast has been produced by Refeyn, who retains sole responsibility for content. About this content.
Speaker
Xiaojing Huang, Facility Manager for Molecular Surface Interaction, University of New South Wales

Xiaojing obtained her bachelor degree in Biochemistry and Master of Science in Chemistry from York University, Toronto, Canada. She recently completed her doctoral research at University of New South Wales, Sydney, Australia. Currently, she is working as a facility manager for Molecular Surface Interaction.
Moderator
Sarah Hiddleston, Nature Research Custom Media

Sarah Hiddleston is a freelance journalist who has worked with Nature Research Custom Media since 2015. Previously, Sarah worked for a decade in Madras (Chennai), India, specialising in health, pharmaceutical and environmental stories. Sarah holds an MA in Investigative Journalism from City University London, an MSc in Political Theory from the London School of Economics, and an undergraduate degree in History from the University of Cambridge, UK.